Method for preparing irbesartan ultrafine particles by applying supercritical anti-solvent technology
A supercritical anti-solvent, ultra-fine particle technology, used in medical preparations containing active ingredients, pharmaceutical formulations, metabolic diseases, etc., can solve the problems of difficult filtration and drying, large density and flow index, poor flowability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
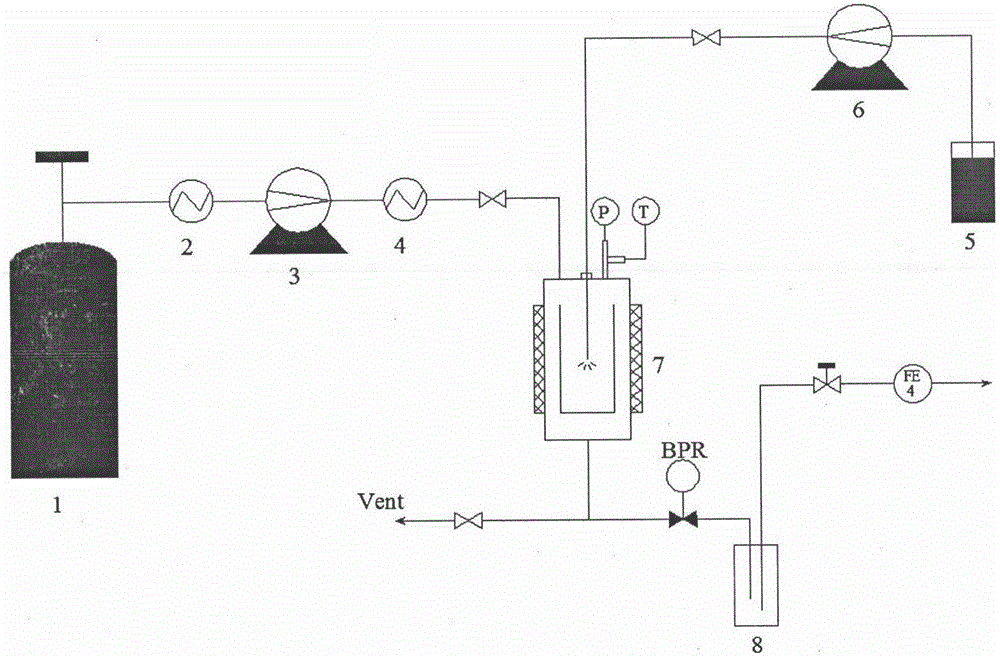
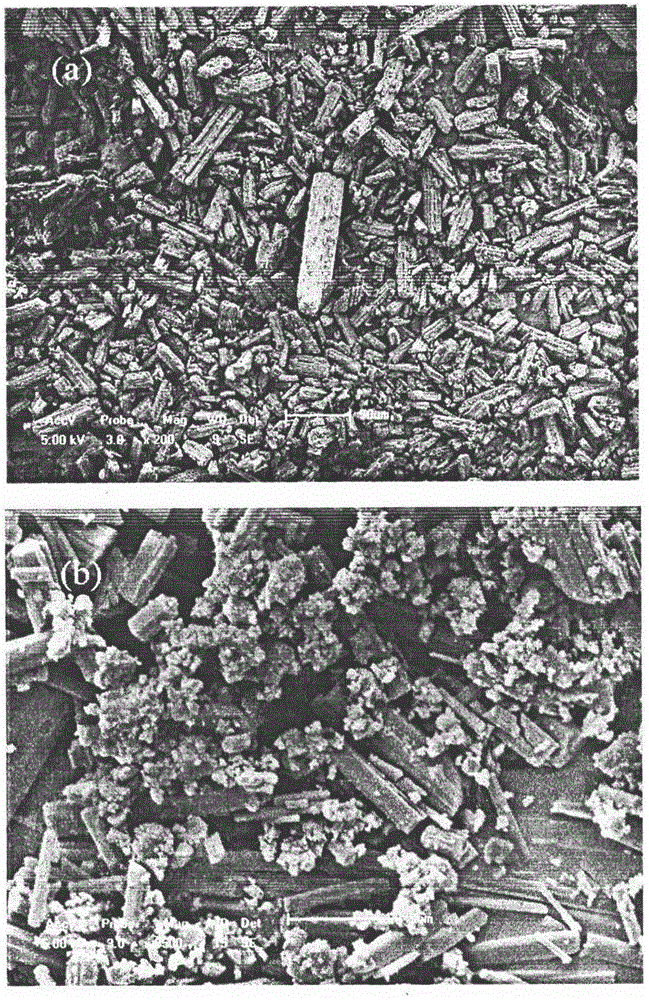
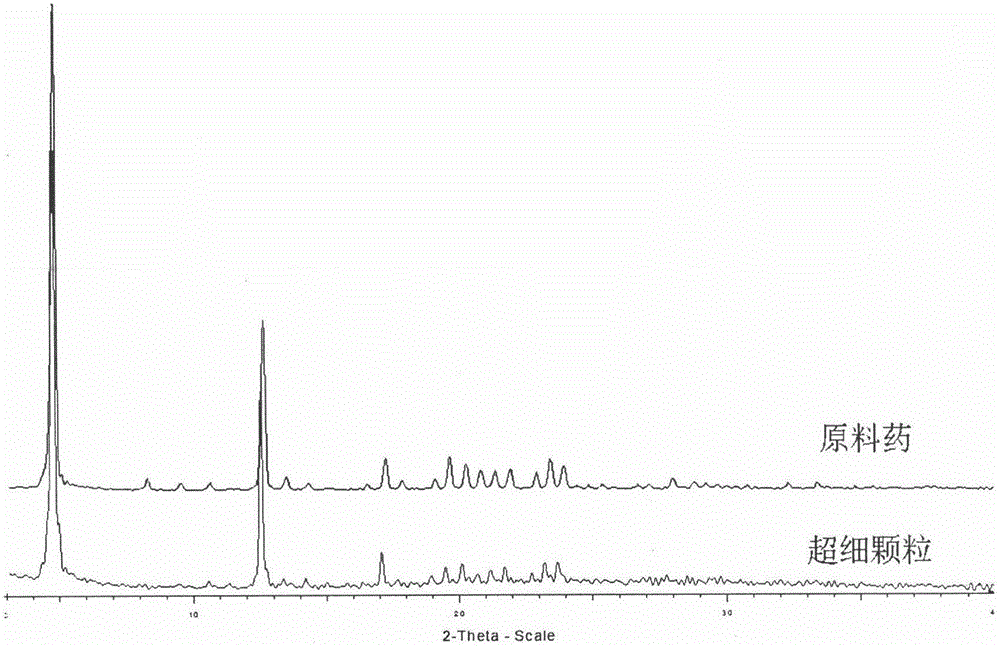
[0033] Accurately weigh 240 mg of irbesartan raw material, add it to a mixed solvent of ethanol: dimethyl sulfoxide = 13 mL: 2 mL, dissolve at 25 ° C, and ultrasonicate at 70 Hz for 5 min to ensure that the solute is completely dissolved; set a supercritical antisolvent The crystallization pressure in the equipment system is 13.5MPa, and the crystallization temperature is 48°C; the drug solution is sprayed into the crystallization kettle at a sampling rate of 0.8mL / min through the HPLC pump, and the supercritical CO 2 The inlet and outlet of the control system is 13.5MPa, in which the supercritical CO 2 The flow rate of the sample is controlled between 5.0-6.0L / min; after the injection volume reaches 10mL, stop the injection and maintain the supercritical CO 2 After 80 minutes of the on and off state, stop the CO 2After the pressure in the system dropped to atmospheric pressure, the sample was taken out from the crystallization kettle for testing and analysis; the obtained ir...
Embodiment 2
[0035] Accurately weigh 225 mg of irbesartan raw material, add it to a mixed solvent of dichloromethane: dimethyl sulfoxide = 13.5 mL: 1.5 mL, dissolve at 25 ° C, and ultrasonicate at 70 Hz for 5 min to ensure that the solute is completely dissolved; set The crystallization pressure in the supercritical antisolvent equipment system is 15MPa, and the crystallization temperature is 45°C; the drug solution is sprayed into the crystallization kettle at a sampling rate of 0.8mL / min through the HPLC pump, and the supercritical CO 2 The inlet and outlet of the control system is 15MPa, in which the supercritical CO 2 The flow rate of the sample is controlled between 5.0-6.0L / min; after the injection volume reaches 10mL, stop the injection and maintain the supercritical CO 2 After 70 minutes of on-off and off-state, stop the CO 2 After the pressure in the system dropped to atmospheric pressure, the sample was taken out from the crystallization kettle for testing and analysis; the obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com