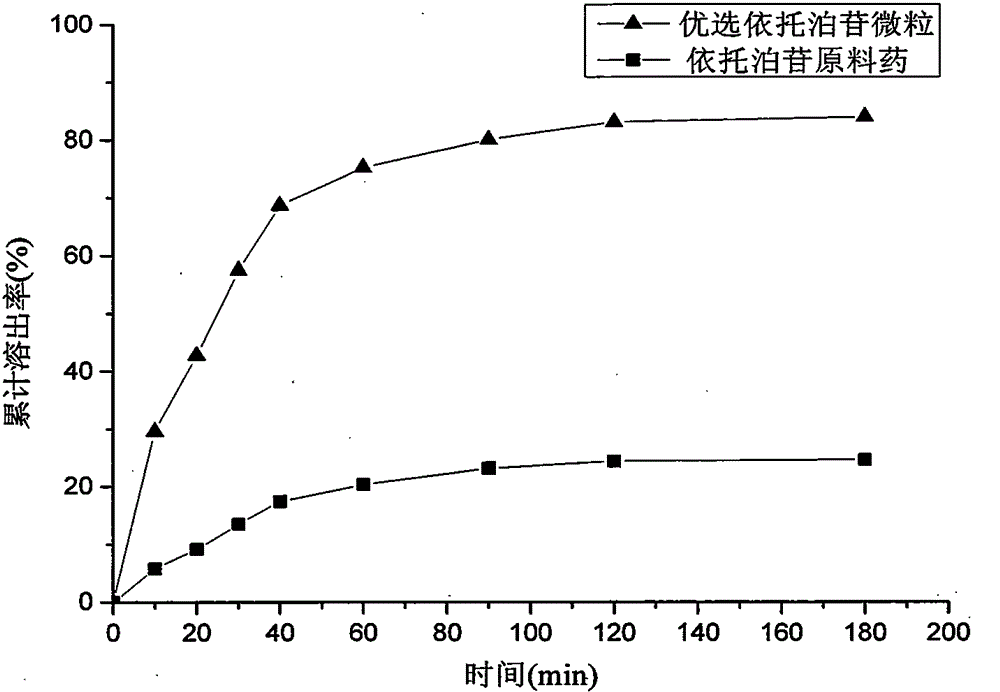
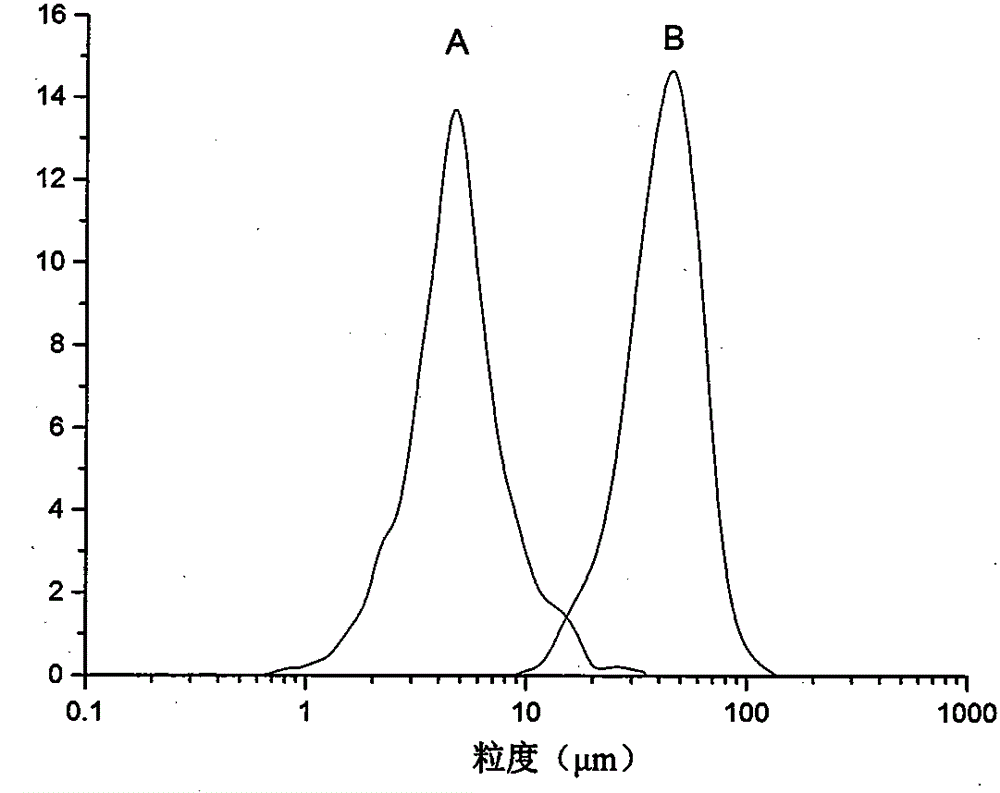
Method for preparing etoposide ultrafine particles by supercutical fluid technology
A technology of etoposide and ultra-fine particles, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, powder delivery, etc. It can solve the problems of organic solvent residues, wide product particle size distribution, variability, etc., and achieve solubility Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
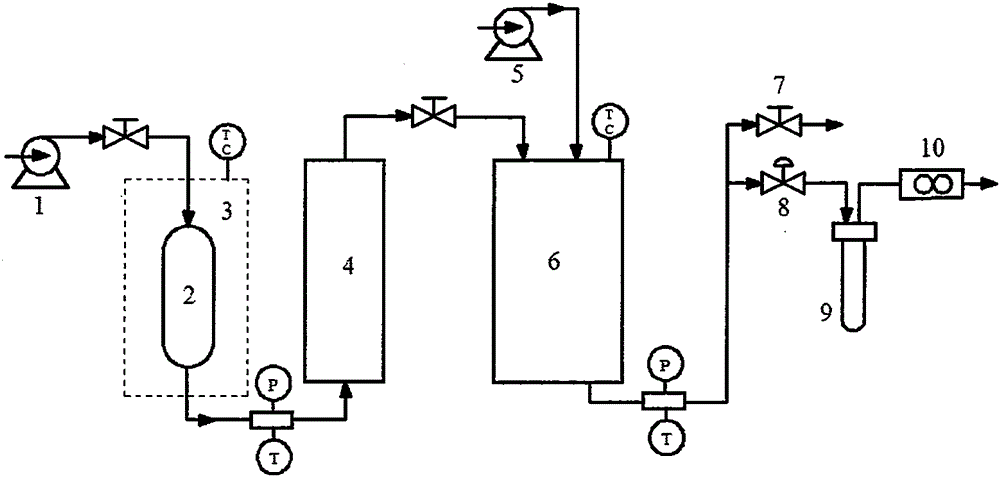
[0028] Weigh 100 mg of etoposide raw material, dissolve in 10 mL of acetone to form a solution. Set the pressure in the crystallization tank to 10MPa, the temperature to 40°C, and remove the air in the tank. After the pressure and temperature are constant, adjust the flow control valve to keep the CO 2 The exhaust speed is 10L / min, start the high-efficiency liquid phase pump, and the solution is input into the crystallization kettle at a speed of 1mL / min. After the solution completely enters the crystallization kettle, keep the gas in and out for more than 30min. Subsequent shutdown of the CO 2 Inlet valve, depressurize and remove sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com