Method for preparing ultrafine drug particles in process of improving supercritical anti-solvent by nonsolvent method
A supercritical anti-solvent, non-solvent technology, applied in solution crystallization and other directions, can solve the problems of drugs that cannot fully exert their curative effect, difficult to absorb, and intense preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
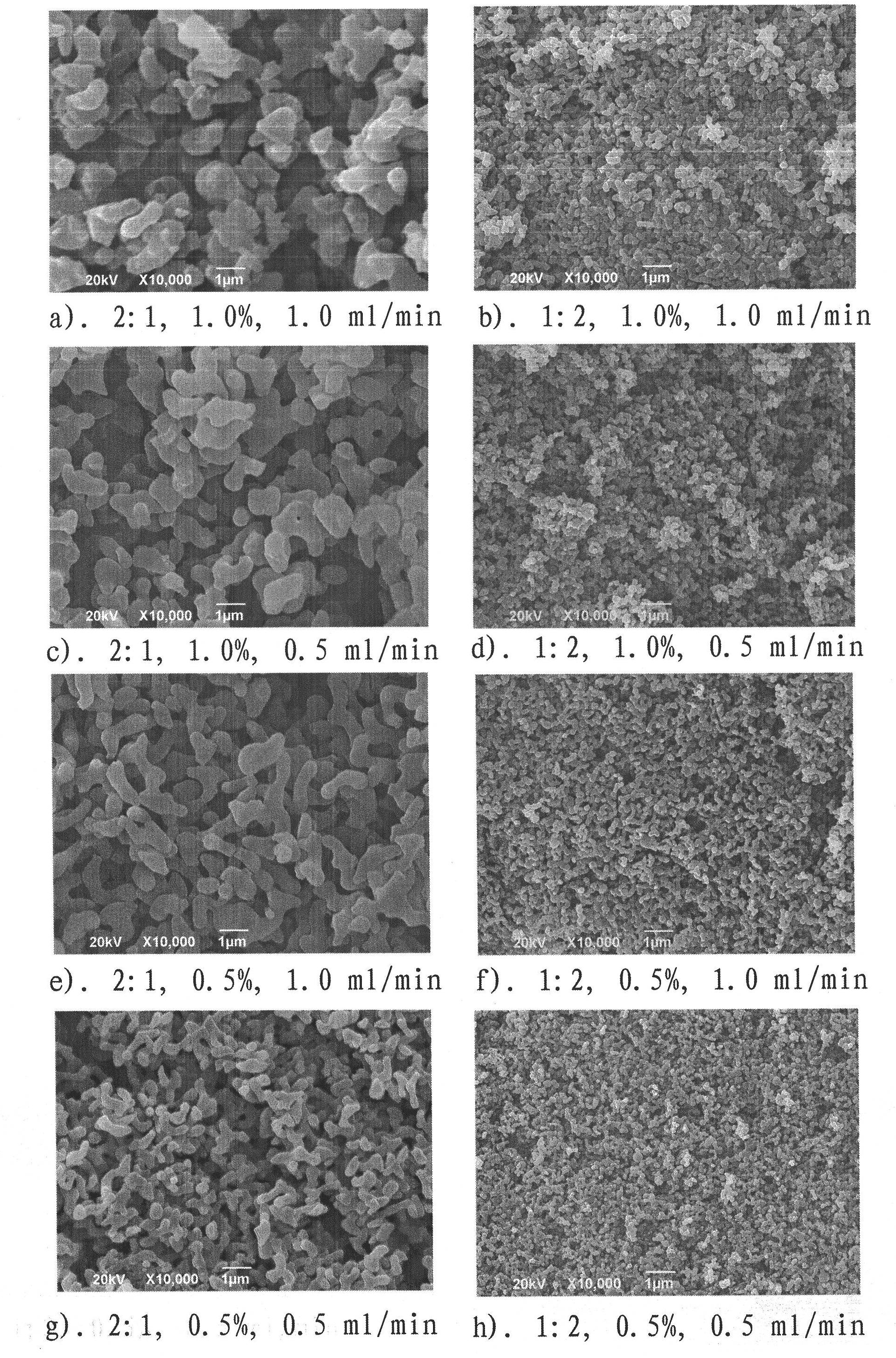
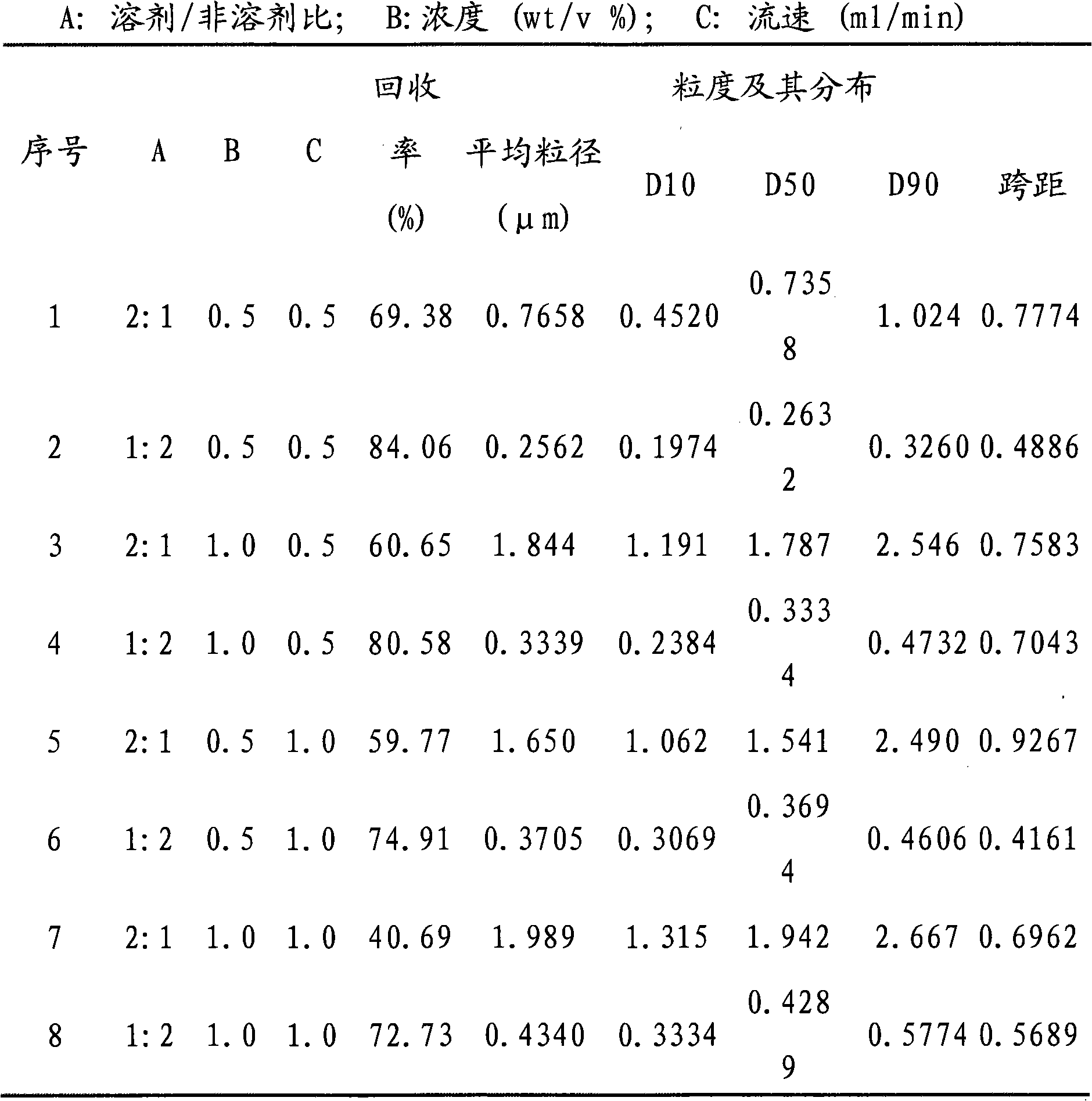
[0021] A full factorial experimental design was used to investigate the influence of factors such as solvent / non-solvent ratio, concentration and flow rate on the miniaturization of puerarin particles by supercritical carbon dioxide anti-solvent method. Such as figure 1 a experimental condition a).2:1, 1.0%, the implementation process of 1.0ml / min is as follows: the puerarin of 300mg is dissolved in the dehydrated alcohol of 20ml, then adds the non-solvent-dichloromethane of 10ml, obtains solvent / The non-solvent ratio is 2:1, and the concentration is 1.0% puerarin solution. After the carbon dioxide in the steel cylinder is liquefied by the refrigeration system, it is pressurized by the high-pressure plunger pump, and then pumped into the autoclave after the temperature is raised by the constant temperature water bath in the pipeline. The air release valve releases air at a certain rate, and adjusts the temperature of the external drying oven and pipeline water bath of the au...
Embodiment 2
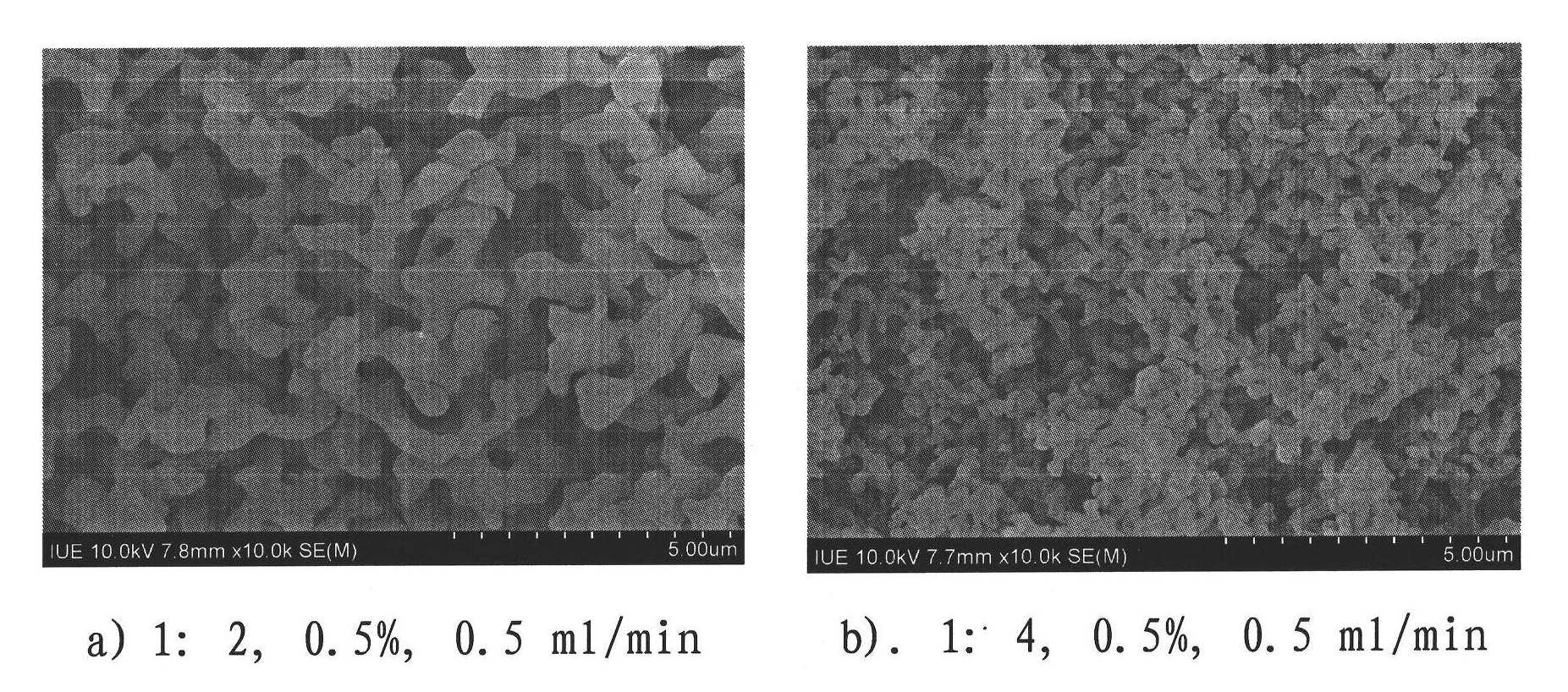
[0026] Dissolve methotrexate in dimethyl sulfoxide, and then add non-solvent acetone to obtain dimethyl sulfoxide / acetone ratios of 1:2 and 1:4, and a concentration of 0.5% methotrexate solution, with a flow rate of 0.5 ml / min is processed with the supercritical carbon dioxide anti-solvent process parameters in Example 1. The morphology of the obtained methotrexate ultrafine particles is as follows: figure 2 As shown, the average particle diameters are 1243nm and 446nm, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com