Composite nanoparticles of polymer coated curcumin eutectic/synergistic components, and preparation and application thereof in pharmacy
A composite nanoparticle and curcumin-coated technology, which is applied to medical preparations with non-active ingredients, polymer compound non-effective ingredients, medical preparations containing active ingredients, etc., can solve the problem of difficult to obtain coated structure nanoparticles, solid Wide particle size distribution, organic solvent residue and other problems, to achieve good solubility, increase yield per unit time, improve dissolution rate and oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
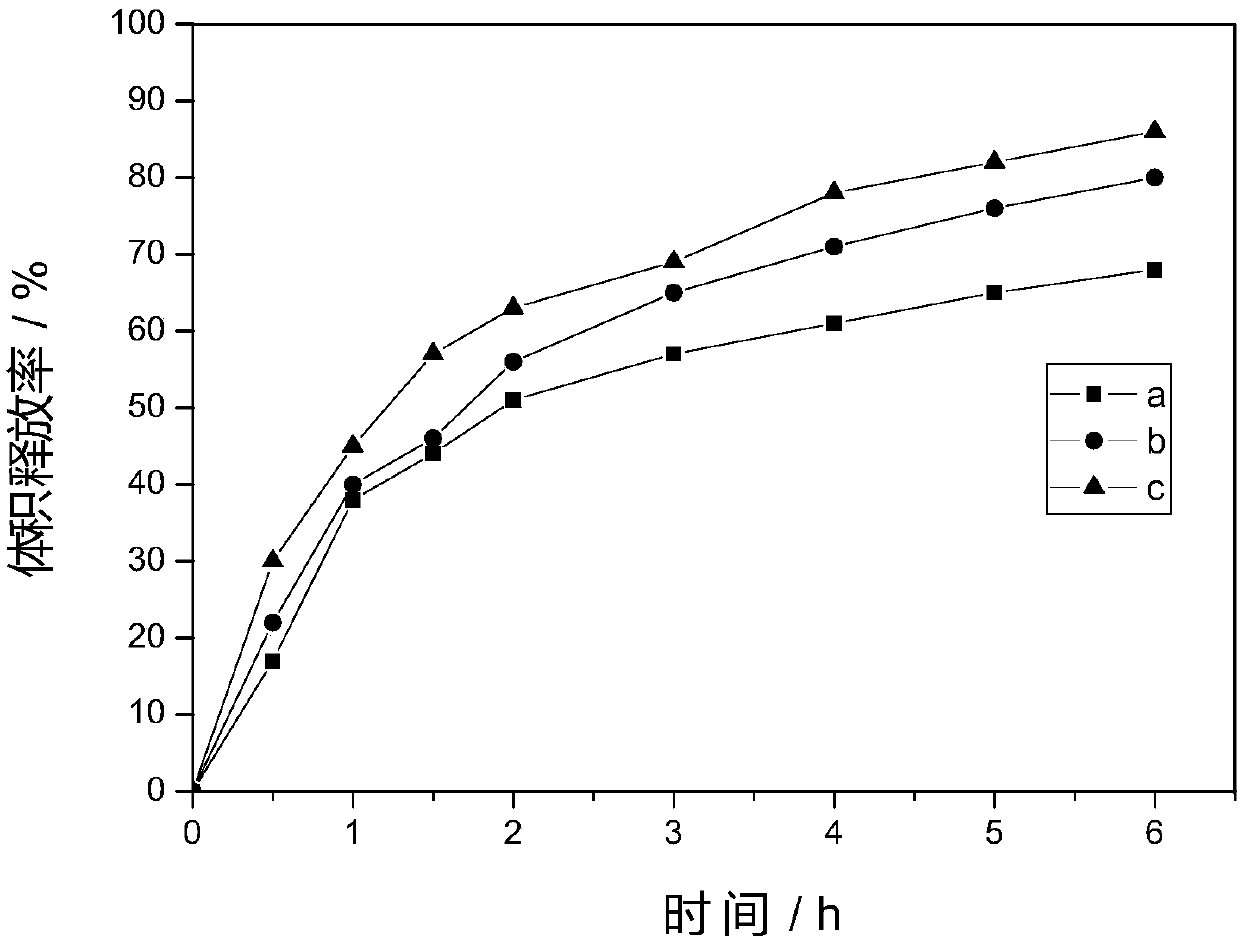
[0059] Disperse the curcumin-2,5-dihydroxybenzoic acid eutectic compound and piperine at a mass ratio of 40:1 in the liquefied CO 2 middle. will CO 2 (containing curcumin-2,5-dihydroxybenzoic acid eutectic compound and piperine) is pumped into the autoclave, and after the temperature of the autoclave reaches 35°C and the pressure is the preset value of 10MPa and stabilizes for 5min, add 0.5ml The flow rate of / min was pumped into an aqueous solution of polyvinylpyrrolidone with a concentration of 5 mg / ml, wherein the mass ratio of polyvinylpyrrolidone and curcumin-2,5-dihydroxybenzoic acid eutectic compound was 5:1. After the solution is pumped, continue to pass through the CO 2 After 0.5h, stop feeding CO 2 , release the pressure, and collect the particles in the reactor to obtain the curcumin eutectic composition nanoparticles. Its average particle diameter is 710nm, and the encapsulation efficiency is 74%.
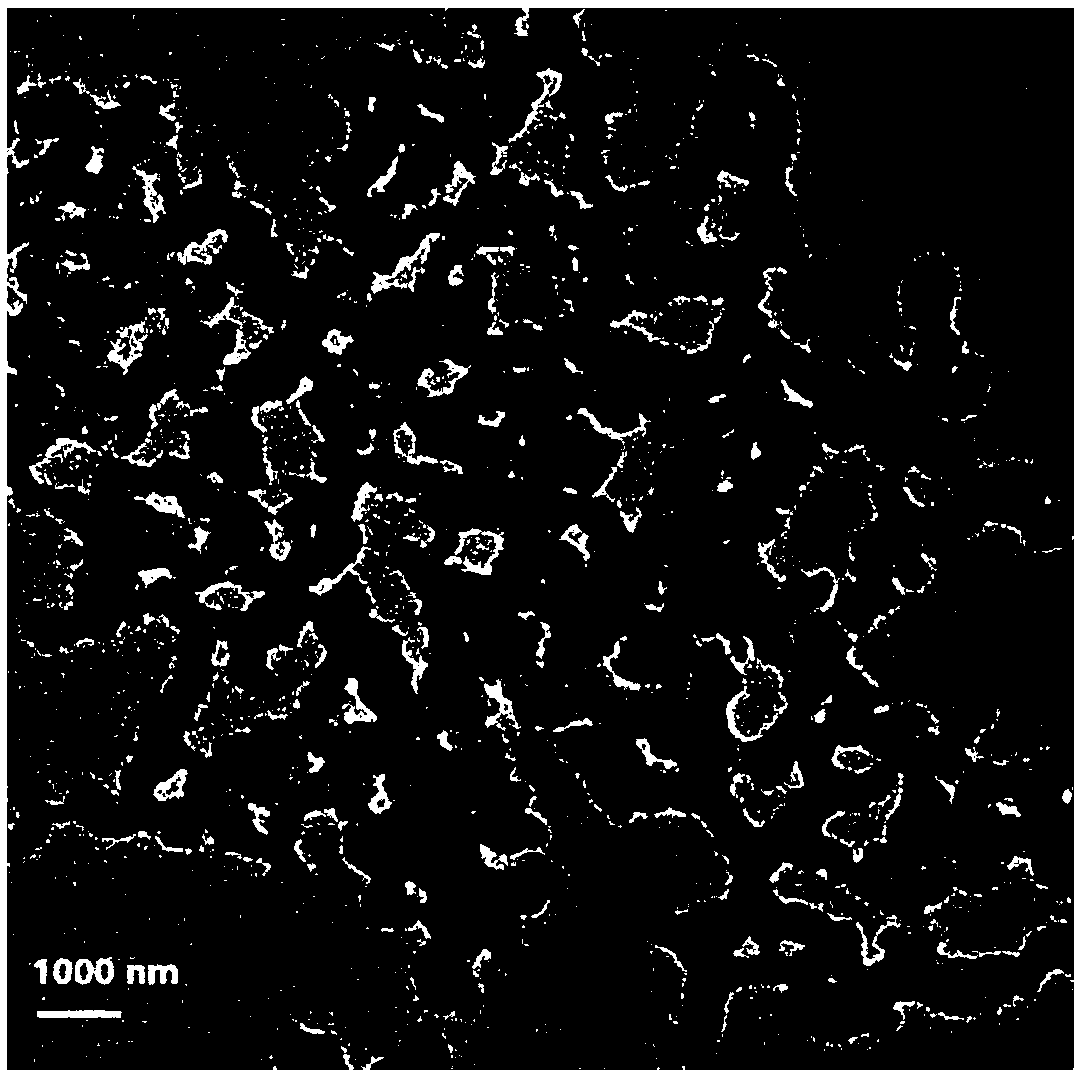
[0060] Morphology analysis of polymer-coated curcumin co-crys...
Embodiment 2
[0063] Disperse the curcumin-2-aminopyridine co-crystal compound and acarbose in the liquefied CO at a mass ratio of 100:1 in the dark 2 middle. will CO 2 (containing curcumin-2-aminopyridine eutectic compound and acarbose) is pumped into the autoclave, and after the temperature of the autoclave reaches 35°C and the pressure is the preset value of 20MPa and stabilizes for 5min, the The flow rate pumped into the poloxamer aqueous solution with a concentration of 20 mg / ml, wherein the mass ratio of poloxamer and curcumin-2-aminopyridine cocrystal compound was 5:1. After the solution is pumped, continue to pass through the CO 2 After 0.5h, stop feeding CO 2 , release the pressure, and collect the particles in the reactor to obtain the curcumin eutectic composition nanoparticles. Its average particle diameter is 690nm, and the encapsulation efficiency is 70%.
Embodiment 3
[0065] Disperse the curcumin-4-aminophenol eutectic compound and piperine in the liquefied CO at a mass ratio of 40:1 in the dark 2 middle. will CO 2 (containing curcumin-4-aminophenol eutectic compound and piperine) is pumped into the autoclave, and after the temperature of the autoclave reaches 50° C., the pressure is the preset value of 10 MPa and stabilizes for 5 minutes, the flow rate of 0.5ml / min Pump in an aqueous solution of hydroxypropyl cyclodextrin with a concentration of 5 mg / ml, wherein the mass ratio of hydroxypropyl cyclodextrin and curcumin-4-aminophenol eutectic compound is 10:1. After the solution is pumped, continue to pass through the CO 2 After 0.5h, stop feeding CO 2 , release the pressure, and collect the particles in the reactor to obtain the curcumin eutectic composition nanoparticles. Its average particle diameter is 530nm, and the encapsulation efficiency is 72%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com