Novel crystal form of gefitinib and preparation method thereof based on supercritical anti-solvent technology
A technology of gefitinib and its crystal form, which is applied in organic chemical methods, bulk chemical production, organic chemistry, etc., can solve the problems of insufficient stability, large dosage, poor water solubility, etc., and achieve easy industrial production and preparation The method is simple and the effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
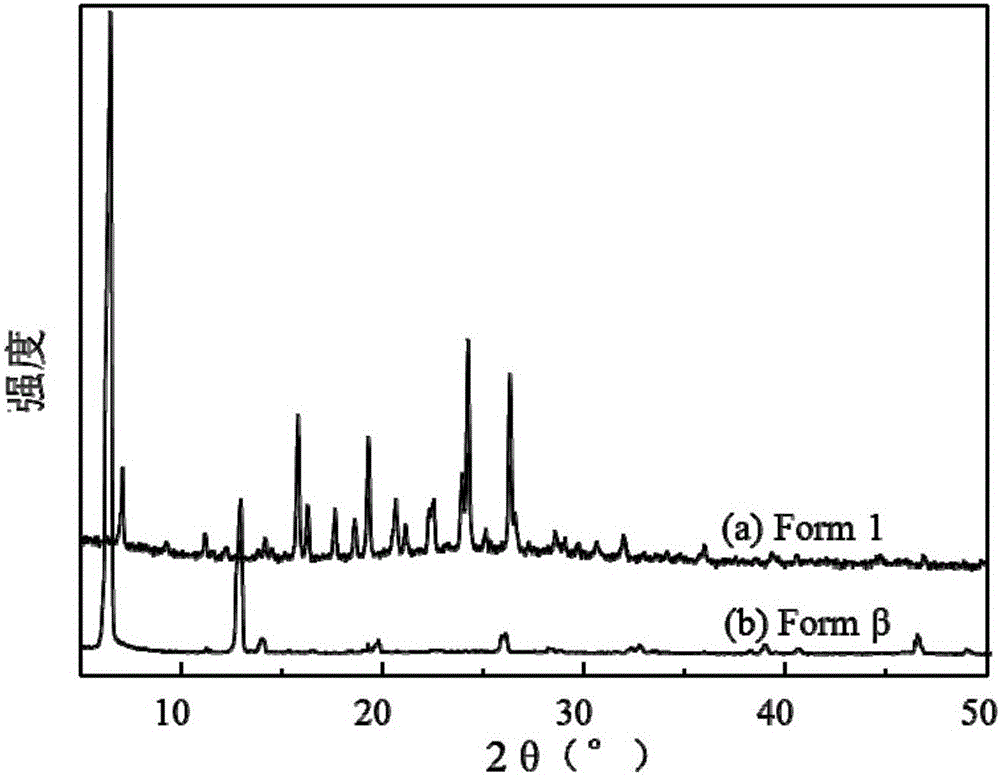
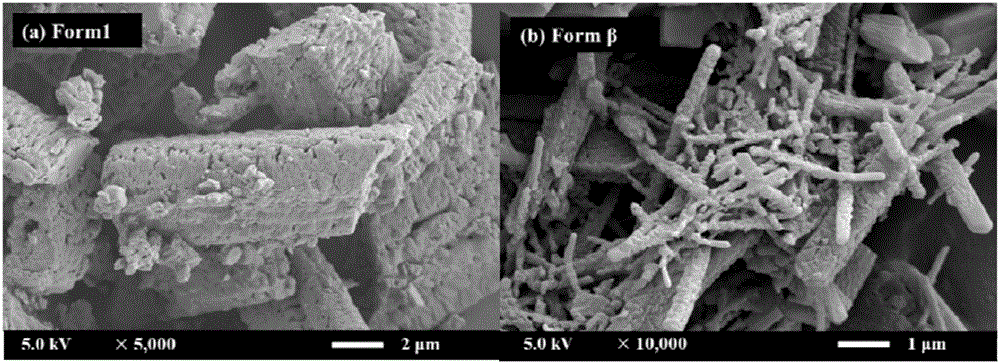
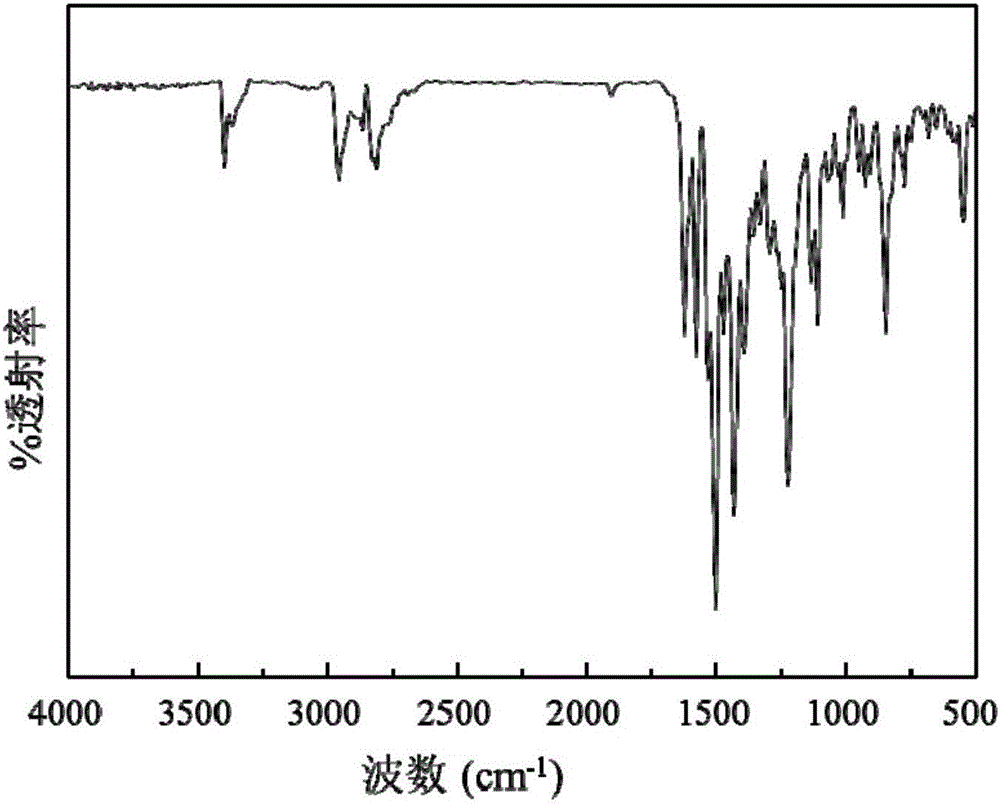
[0049] A preparation of gefitinib crystal form β: accurately measure 40mL of DCM and 160mL of EtOH and configure it into a mixed solvent, accurately weigh 100mg of gefitinib powder (the bulk drug crystal form is Form 1), and dissolve it in the above 100 mL of mixed solvent was prepared into a gefitinib solution with a concentration of 1 mg / mL, and the remaining mixed solvent was used for later use. Before starting, set the temperature of the autoclave to 40°C and the pressure to 90bar. Open the steel cylinder, use a high pressure pump to introduce carbon dioxide from the top of the settling kettle at a flow rate of 20 g / min, when the temperature and pressure in the kettle reach the above set values, use another high pressure pump to transfer the remaining mixed solvent of DCM and EtOH to The rate of 1.0mL / min was passed into the sedimentation kettle. After 15min, the system in the reaction kettle reached an equilibrium state, and the introduction of the mixed solvent was stopp...
Embodiment 2
[0053] A preparation of gefitinib crystal form β: accurately measure 200 mL of EtOH as a solvent, accurately weigh 200 mg of gefitinib powder, and dissolve it in 100 mL of solvent to prepare a 2 mg / mL solution, and the remaining solvent is to be prepared. Use; set the temperature of the settling kettle to be 40°C, the pressure to be 90bar, and the flow rate of carbon dioxide injected into the settling kettle is 20g / min. Open the steel cylinder, and use a high-pressure pump to pass carbon dioxide into the settling kettle. When the temperature and pressure in the kettle reach the above set values, use another high-pressure pump to pass the solvent into the settling kettle at a rate of 0.5 mL / min. After 15 minutes, stop the flow of the solvent. into the solvent, and the gefitinib solution was injected at the same rate. After the sample injection was completed, carbon dioxide was continued to be introduced for 40 min, the carbon dioxide pump was stopped, the pressure of the sedime...
Embodiment 3
[0056] A preparation of gefitinib crystal form β: accurately measure 40 mL of DMSO and 160 mL of EtOH to prepare a mixed solvent, accurately weigh 200 mg of gefitinib powder, and dissolve it in 100 mL of the above mixed solvent to configure a concentration of 2 mg / mL of gefitinib solution, and the remaining mixed solvent for use. Before starting, set the temperature of the settling kettle to be 40° C., the pressure to be 90 bar, and the flow rate of carbon dioxide injected into the settling kettle to be 20 g / min. Open the steel cylinder, and use a high-pressure pump to pass carbon dioxide into the settling kettle. When the temperature and pressure in the kettle reach the above set values, use another high-pressure pump to pass the mixed solvent into the settling kettle at a rate of 0.5 mL / min. After 15 minutes, stop The mixed solvent was passed through, and the gefitinib solution was injected at the same rate. After the sample injection was completed, carbon dioxide was cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com