Amlodipine- and erbesartan-containing compound preparation for treating hypertension
A technology of amlodipine and compound preparations, which is applied in the field of compound preparations containing amlodipine and irbesartan for the treatment of hypertension, and can solve the problems of non-optimized proportioning, poor patient compliance, and low blood pressure control rate. Achieve the effects of reducing adverse reactions, quick results, and high blood pressure compliance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
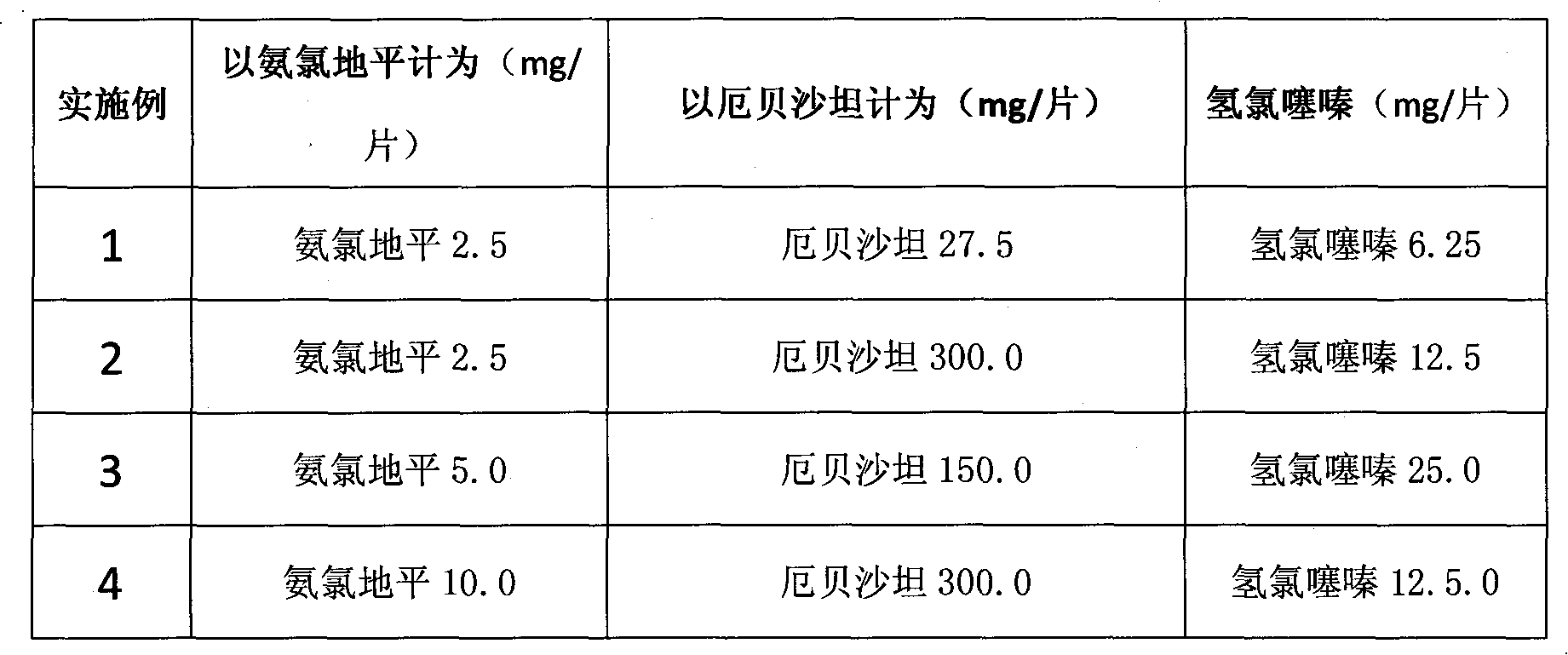
Embodiment 1-4
[0010] Embodiment 1-4 list is as follows:
[0011] In the embodiment, the conventional method in the pharmaceutical industry is used, and the formula in the following table is prepared into tablets, and the pharmaceutically acceptable carrier-adjuvant used for making the tablet is a conventional adjuvant in the art. Wherein the proportioning of its main components is as follows:
[0012]
[0013] Simultaneously, use conventional method in the pharmaceutical industry in the embodiment, can also be prepared into capsule, soft capsule, granule, powder, sustained and controlled release agent or injection dosage form, pharmaceutically acceptable carrier-the auxiliary material used is the art of this dosage form conventional excipients.
Embodiment 5
[0014] Embodiment 5: The curative effect test of the compound preparation of the present invention for treating hypertension:
[0015] The following will illustrate the therapeutic effect of the pharmaceutical composition of the present invention on hypertension through human pharmacodynamic experiments.
[0016] 1. General information:
[0017] Among the 400 clinical cases of hypertensive patients, 140 were 40-50 years old; 140 were 51-60 years old, and 120 were 61-70 years old.
[0018] 2. Diagnostic criteria: According to the "Guidelines for the Prevention and Treatment of Hypertension in China", hypertension is defined as: systolic blood pressure ≥ 140mmHg and / or diastolic blood pressure ≥ 90mmHg without taking antihypertensive drugs.
[0019] 3. Therapeutic method: 400 hypertensive patients are randomly divided into 4 groups, and each group adopts the compound preparation medicine obtained in Examples 1-4 of the present invention respectively to make tablets and take it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com