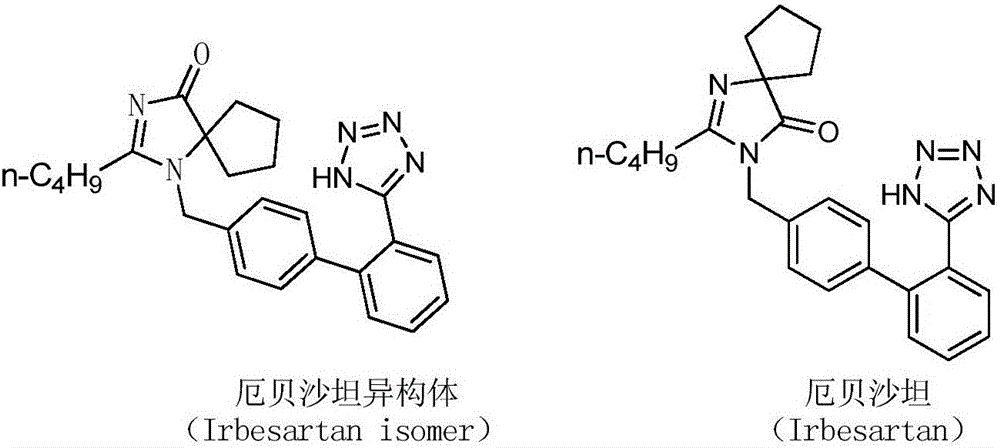
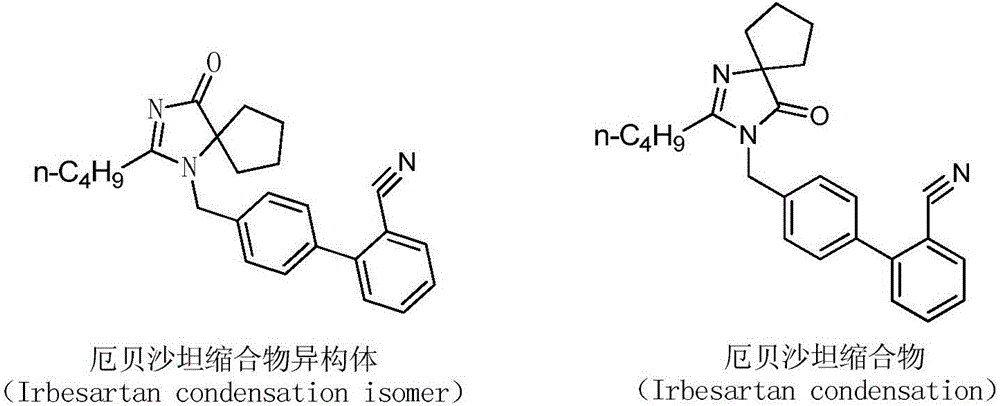
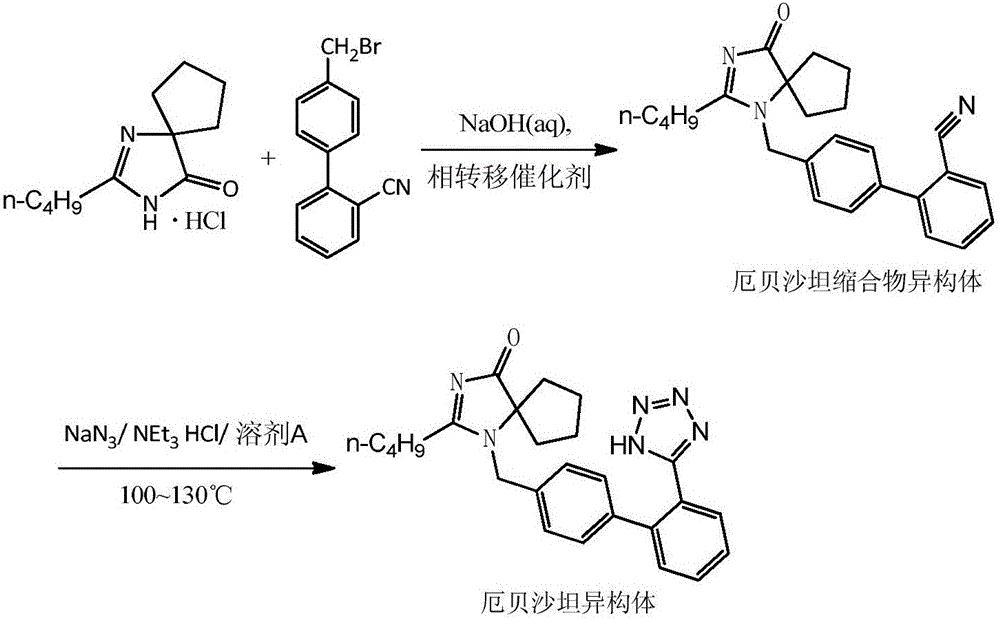
Preparation method of irbesartan isomer and irbesartan intermediate
A technology of irbesartan and isomers, which is applied in the field of preparation of irbesartan isomers and their intermediates, can solve the problems of reporting impurities of irbesartan isomers and preparation methods of their intermediates, etc. Achieve environmental friendliness, low energy consumption and cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 70.0g irbesartan cyclic compound hydrochloride, 82.0g biphenyl bromide, 2.0g tetrabutylammonium bromide and 300mL cyclohexane into a 500mL three-necked flask, and add 240g 30% sodium hydroxide solution at 5°C . Raise the temperature of the material solution to 30° C. and stir for 10 hours to react, and the reaction of the material is complete. The reaction solution was filtered, and the filter cake was separated by column chromatography (using ethyl acetate as eluent) to obtain 40.9 g of irbesartan condensate isomers, with a yield of 35% and a purity of 99.1% by HPLC;
[0030] Add 20g of irbesartan condensate isomers, 14.2g of triethylamine hydrochloride, 6.9g of sodium azide and 125mL of xylene into a 250mL three-neck flask, raise the temperature to 125°C and react for 20-25 hours, and the raw materials are completely reacted . Add 45g of 30% sodium hydroxide solution and 35mL of ethyl acetate to the reaction solution, stir, let stand, and separate the organic la...
Embodiment 2
[0032] Add 70.0g irbesartan cyclic compound hydrochloride, 78.5g biphenyl bromide, 2.0g tetrabutylammonium bromide and 300mL cyclohexane into a 500mL three-necked flask, and add 180g 30% sodium hydroxide solution at 5°C . Raise the temperature of the material solution to 30° C. and stir for 10 hours to react, and the reaction of the material is complete. The reaction solution was filtered, and the filter cake was separated by column chromatography (using ethyl acetate as the eluent) to obtain 37.4 g of irbesartan condensate isomers, with a yield of 32% and a HPLC purity of 99.3%;
[0033] Add 20g of irbesartan condensate isomers, 14.2g of triethylamine hydrochloride, 6.9g of sodium azide and 100mL of xylene into a 250mL three-neck flask, raise the temperature to 125°C and react for 20-25 hours, and the raw materials are completely reacted . Add 45g of 30% sodium hydroxide solution and 35mL of ethyl acetate to the reaction solution, stir, let stand, and separate the organic l...
Embodiment 3
[0035] Add 70.0g irbesartan cyclic compound hydrochloride, 82.0g biphenyl bromide, 2.0g tetrabutylammonium bromide, 100mL tert-butyl acetate and 100mL n-hexane into a 500mL three-necked flask, add 135g 30 % sodium hydroxide solution. Raise the temperature of the feed liquid to 30° C. and stir for 8 hours to react, and the raw materials are completely reacted. The reaction solution was filtered, and the filter cake was separated by column chromatography (using ethyl acetate as the eluent) to obtain 27.2 g of irbesartan condensate isomers, with a yield of 23.2% and a purity of 99.4% by HPLC;
[0036] Add 20g of irbesartan condensate isomers, 14.2g of triethylamine hydrochloride, 6.9g of sodium azide and 125mL of xylene into a 250mL three-neck flask, raise the temperature to 125°C and react for 20-25 hours, and the raw materials are completely reacted . Add 45g of 30% sodium hydroxide solution and 35mL of ethyl acetate to the reaction solution, stir, let stand, and separate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com