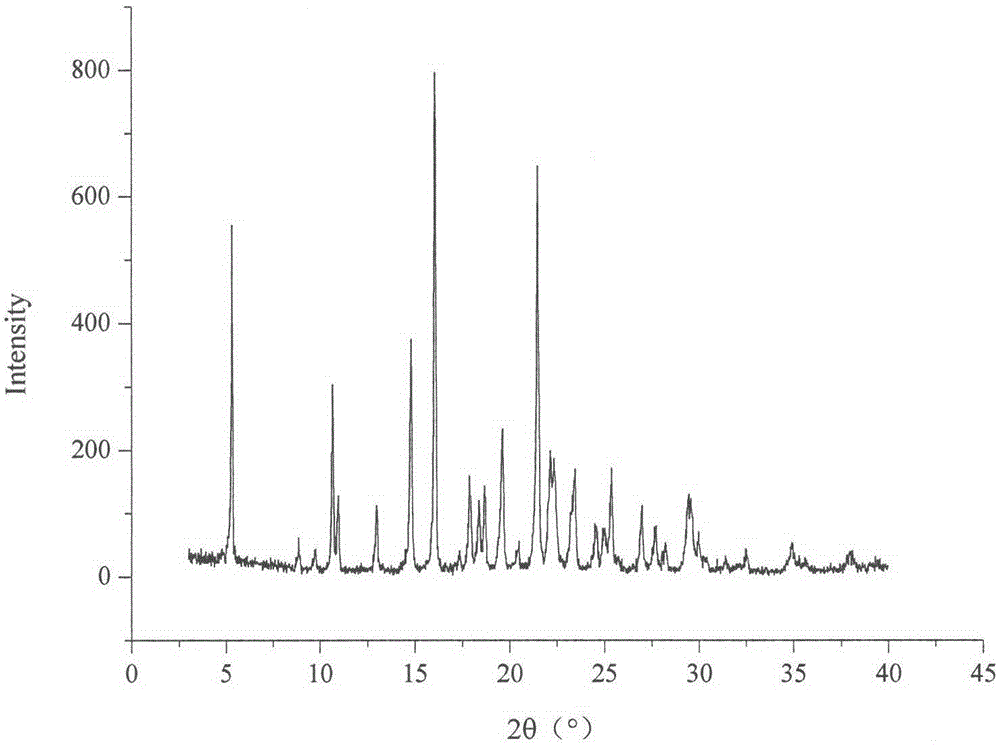
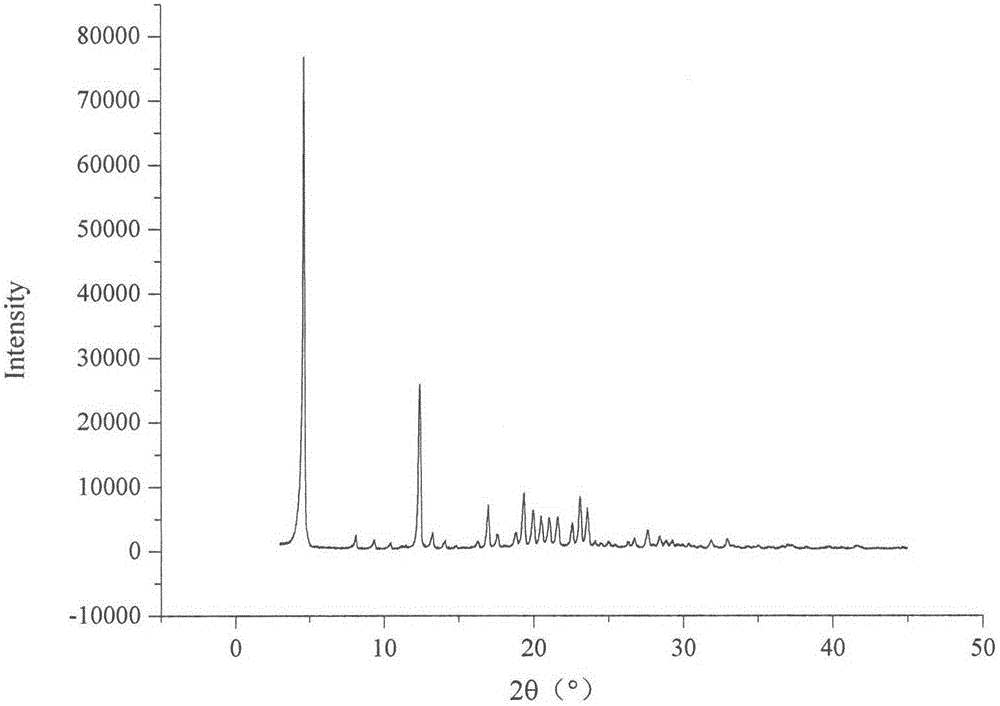
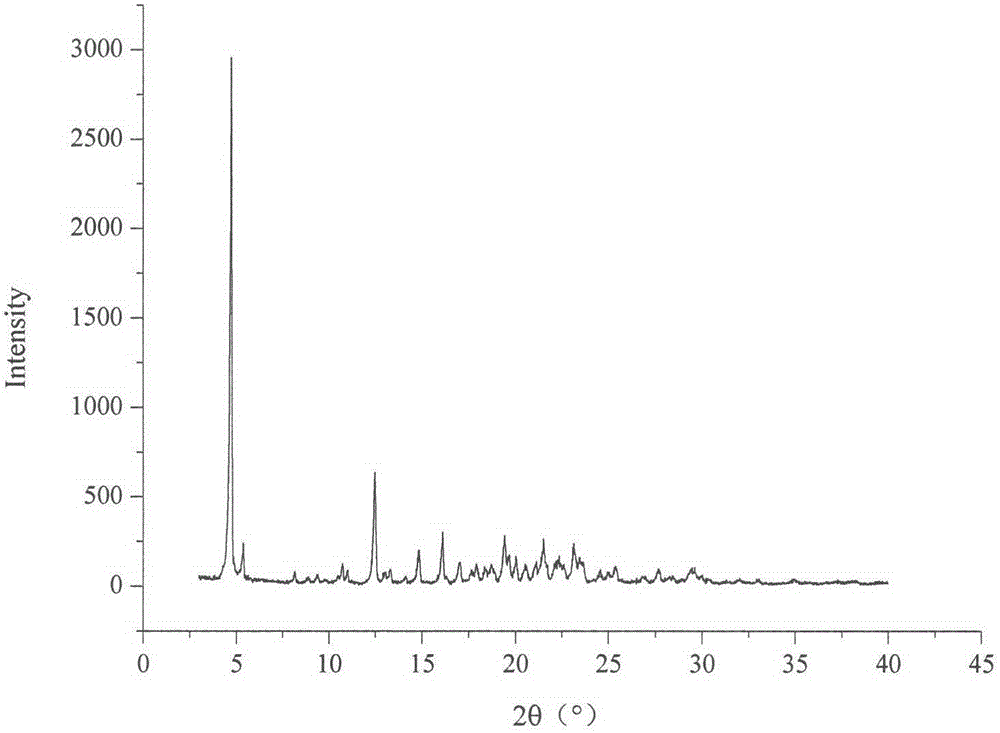
Celecoxib and irbesartan coamorphous substance
An amorphous substance, celecoxib technology, applied in the field of medicine, can solve problems such as difficulty in preparing ideal solid dosage forms, low bulk density, and poor compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Preparation of co-amorphous celecoxib irbesartan
[0063] Add 0.300g celecoxib and 0.339g irbesartan into 50ml of acetonitrile, stir at room temperature (20±5°C) to obtain a clear solution, then evaporate the solvent under reduced pressure at 55°C, and dry in vacuum at 25°C for 24h , to obtain 0.541 g of white powder.
Embodiment 2
[0064] Example 2: Preparation of co-amorphous celecoxib irbesartan
[0065] Add 0.300g celecoxib and 0.339g irbesartan into 50ml of methanol, stir at room temperature (20±5°C) to obtain a clear solution, then evaporate the solvent under reduced pressure at 55°C, and dry in vacuum at 25°C for 24h , to obtain 0.585 g of white powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com