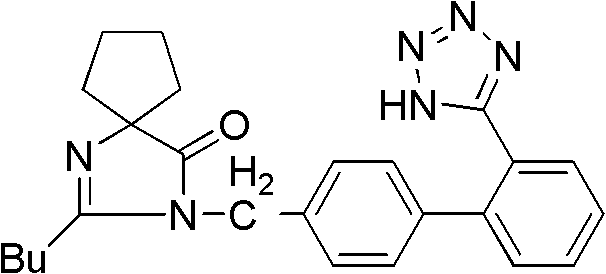
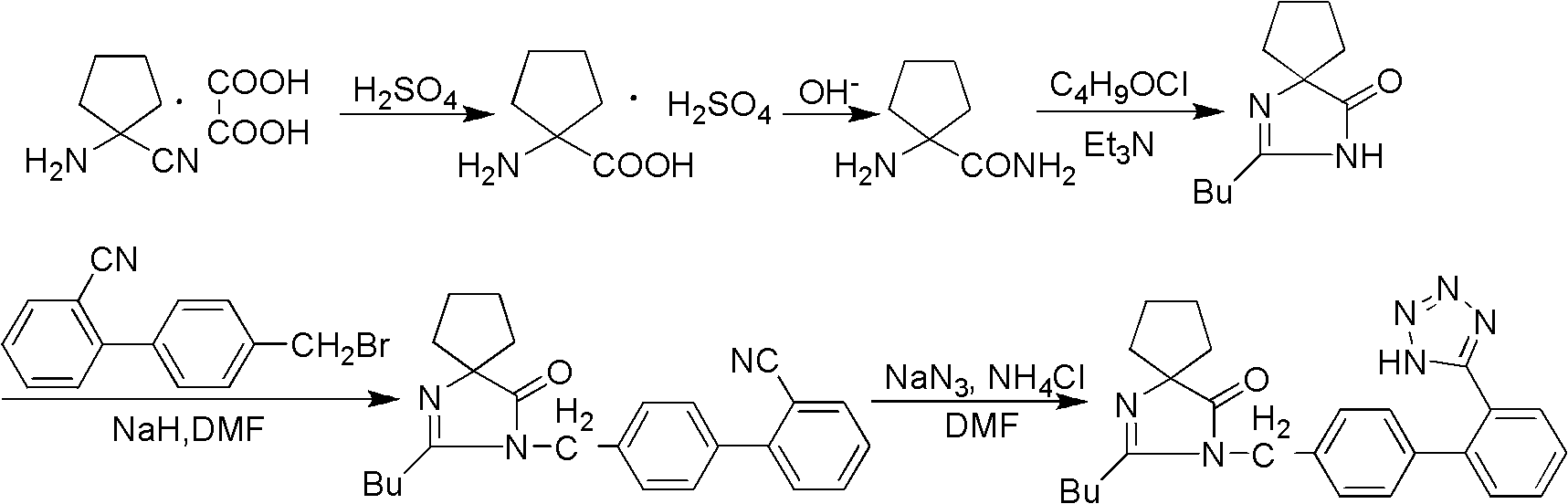
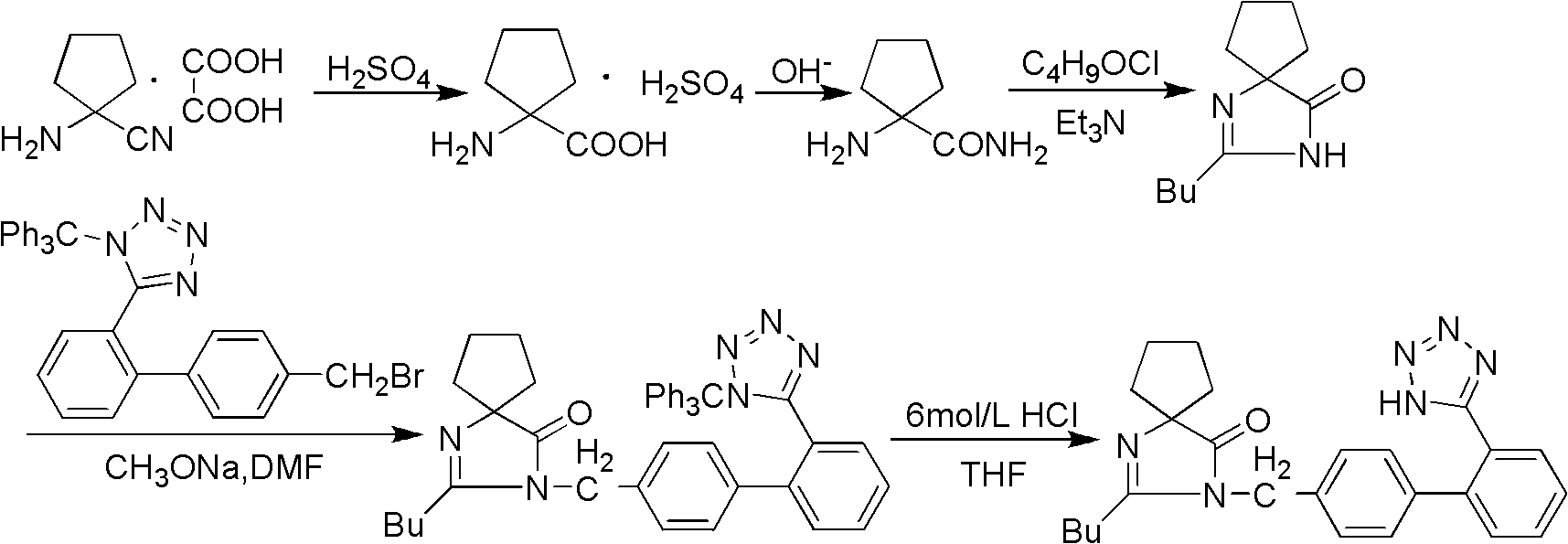
Synthetic method for irbesartan
A synthesis method and intermediate technology, applied in the field of synthesis of antihypertensive drugs, can solve problems such as complex operation steps, high production cost, and complicated operation, and achieve the effects of simplified operation process, increased yield, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The invention will be further described below in conjunction with the examples, and the non-limiting examples are as follows.
[0031] Preparation of intermediate (I) 2-butyl-1,3-diazaspiro[4,4]nonan-1-en-4-one:
[0032] Put 16.5g (0.15mol) of aminocyclopentanitrile in a 250mL flask, add 100mL THF and mechanically stir until the raw material is dissolved, add dropwise a THF solution of 18.0g (0.15mol) of valeryl chloride under stirring, continue stirring for 0.5h, add water 20mL, 50mL of methanol and 20g of potassium hydroxide, reflux for 5h, concentrate under reduced pressure, add water and ethyl acetate to the concentrate for extraction, dry the organic phase with anhydrous magnesium sulfate, filter, and concentrate the filtrate to obtain 27.0g of an oily substance. The rate is 92.7%.
[0033] Intermediate (II) 2-butyl-3-[(2′-cyanobiphenyl-4-yl)methyl]-1,3-diazaspiro[4,4]non-1-ene- Preparation of 4-keto:
[0034] Intermediate (I) 19.4g (0.1mol), 4'-(bromomethyl)-2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com