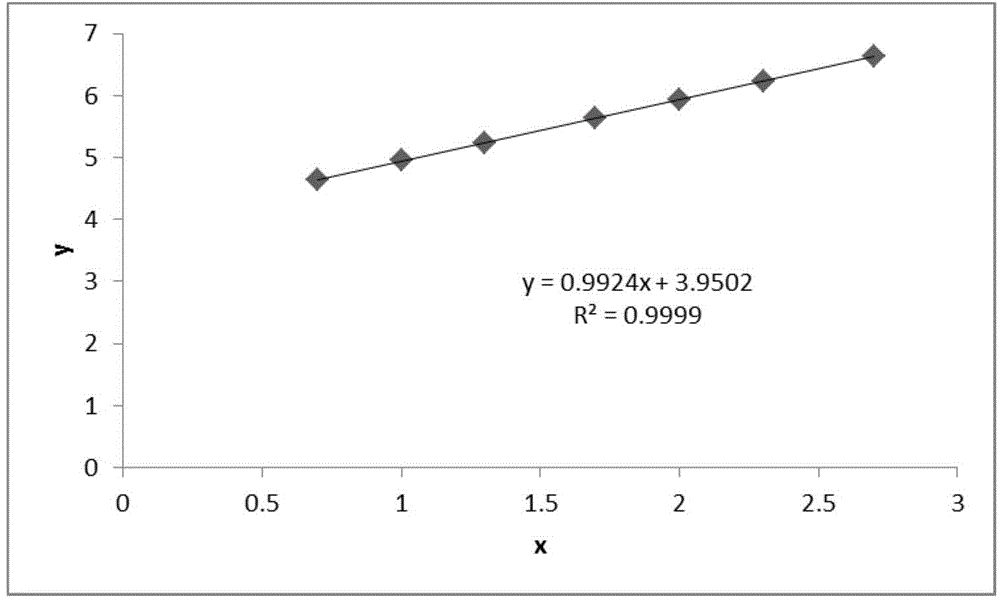
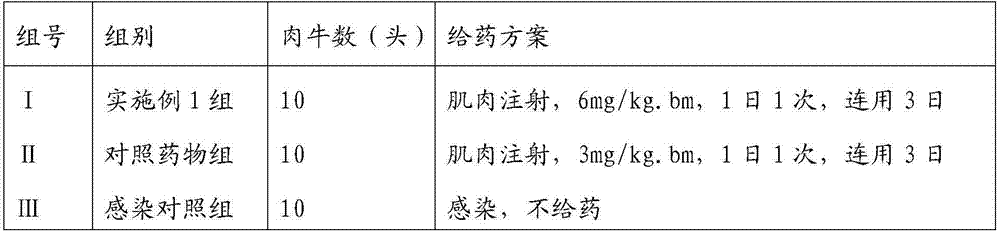
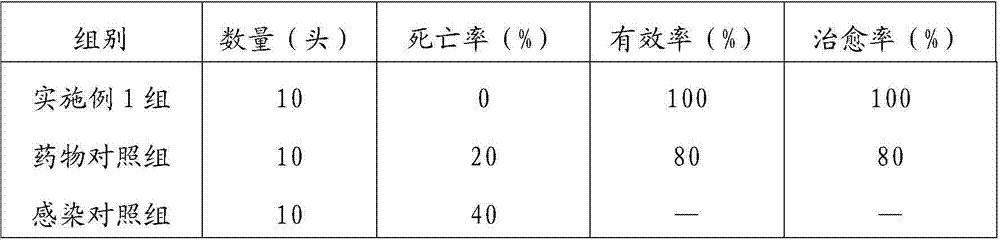
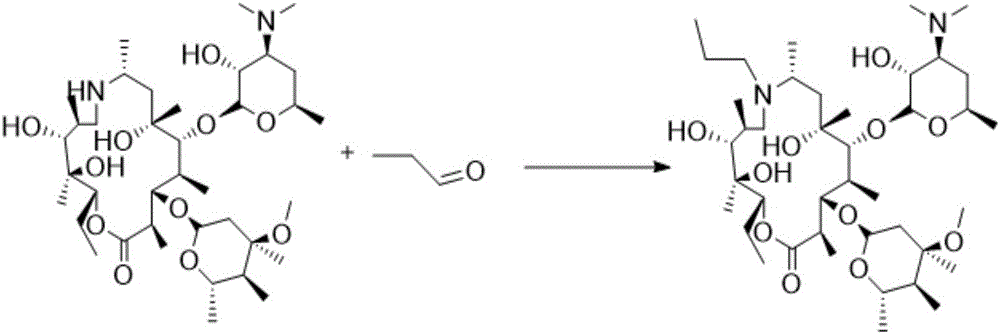
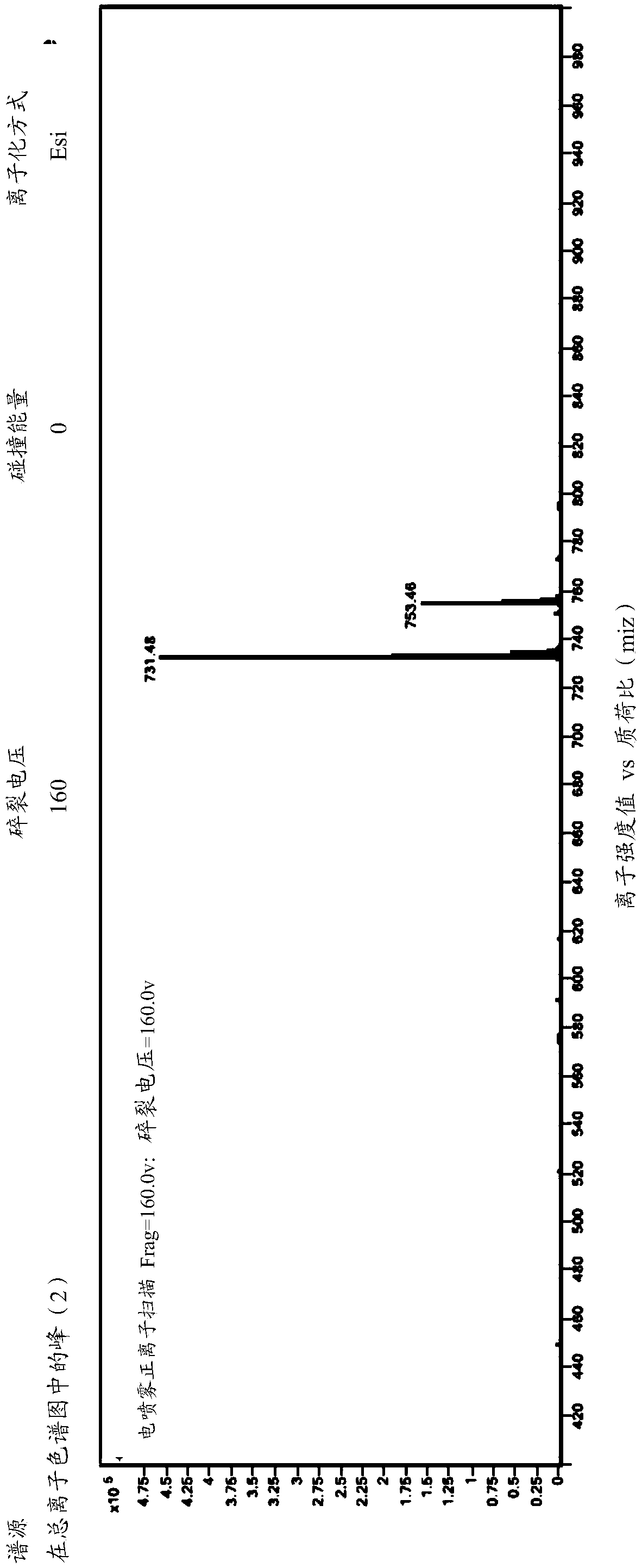
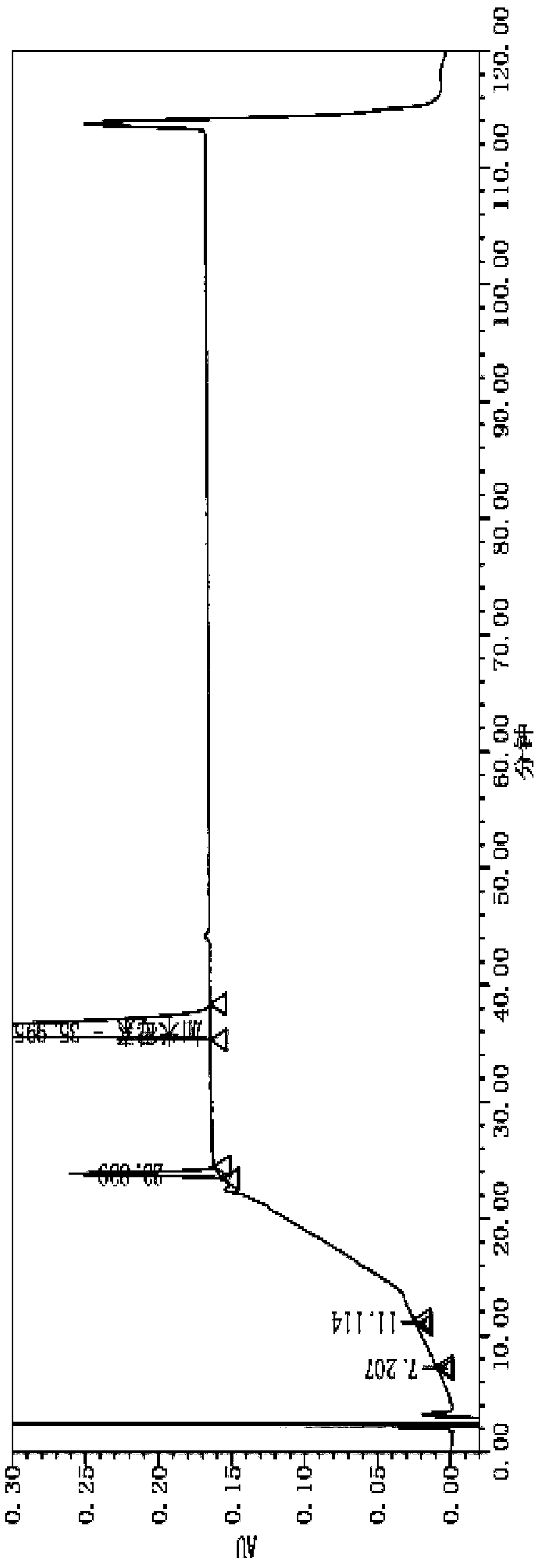
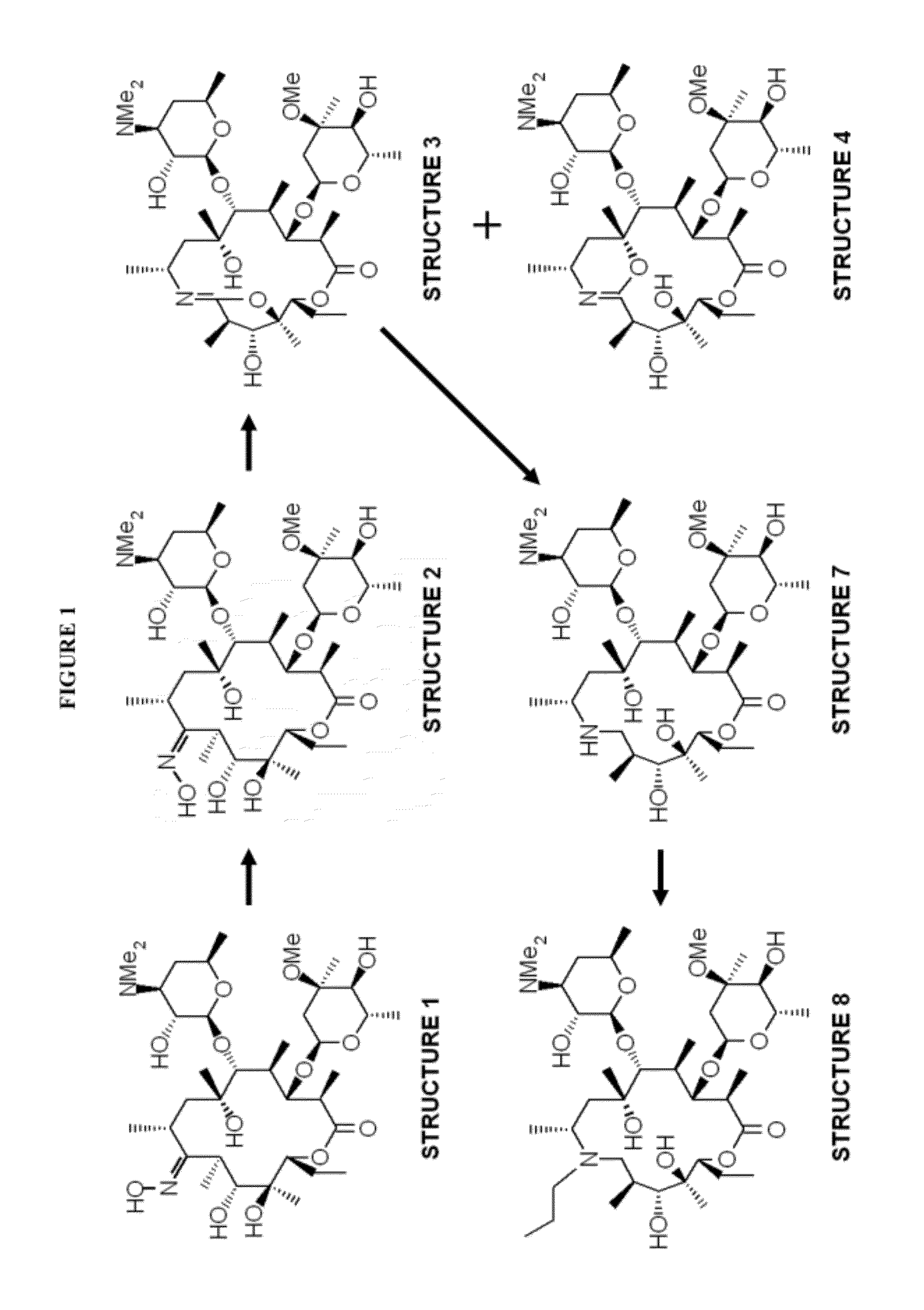
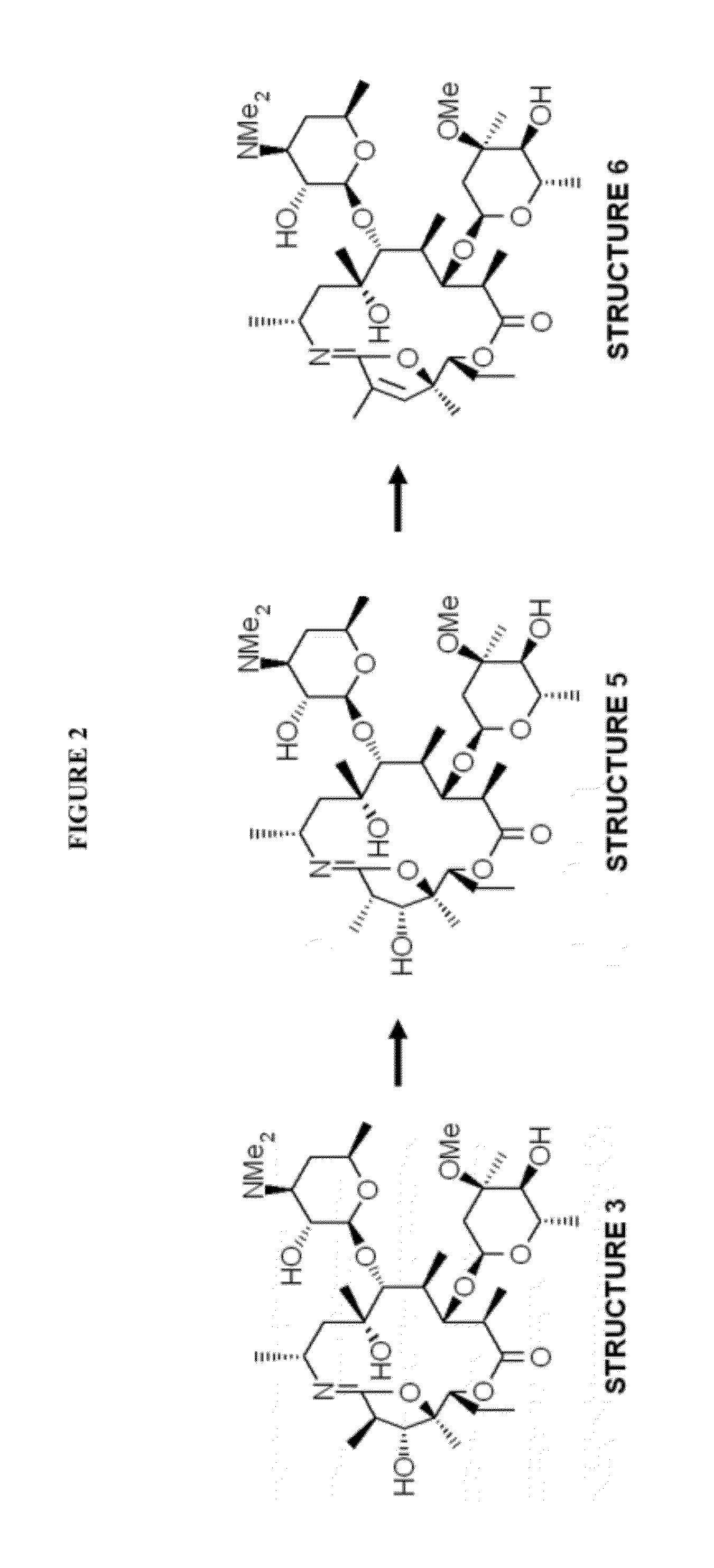
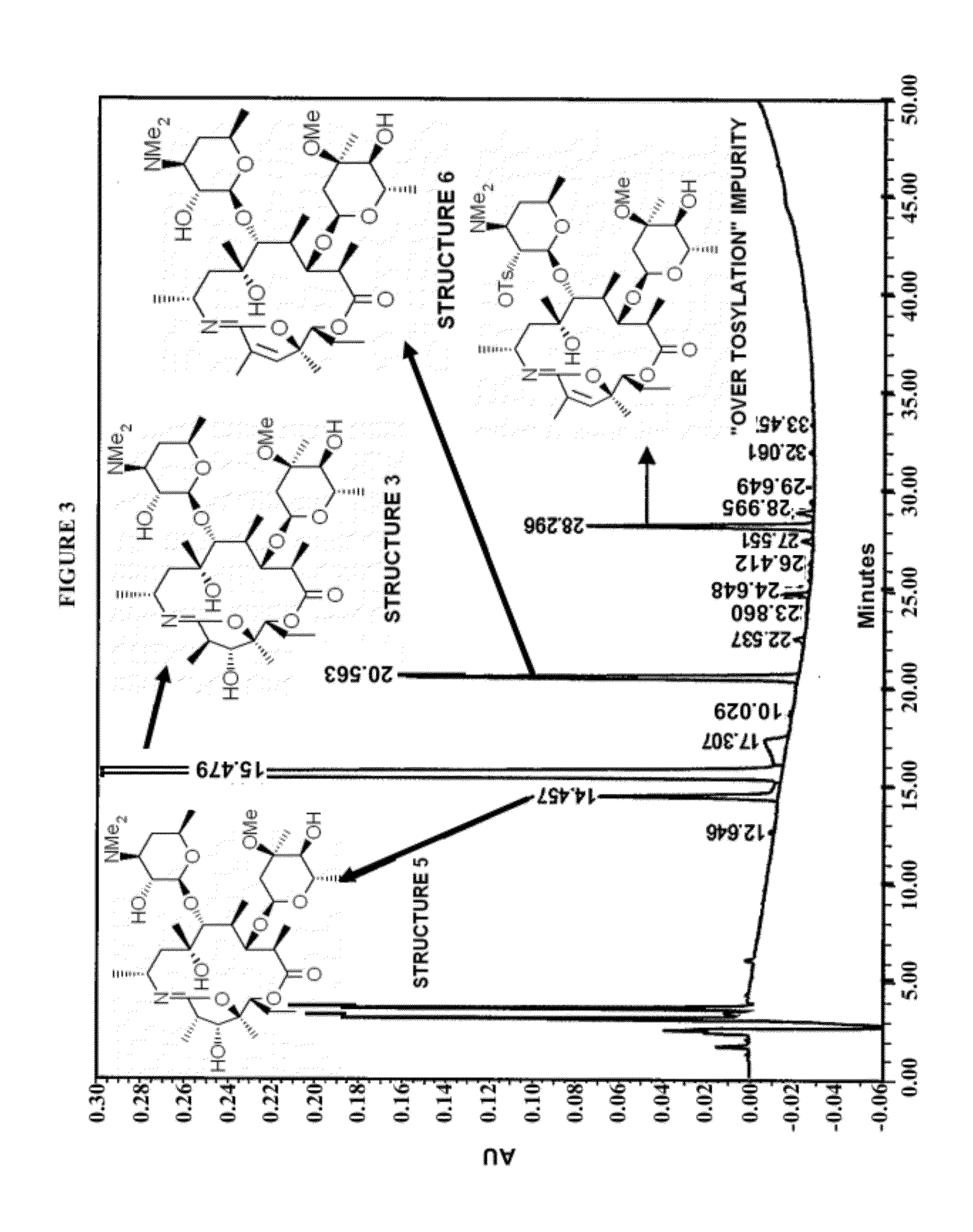
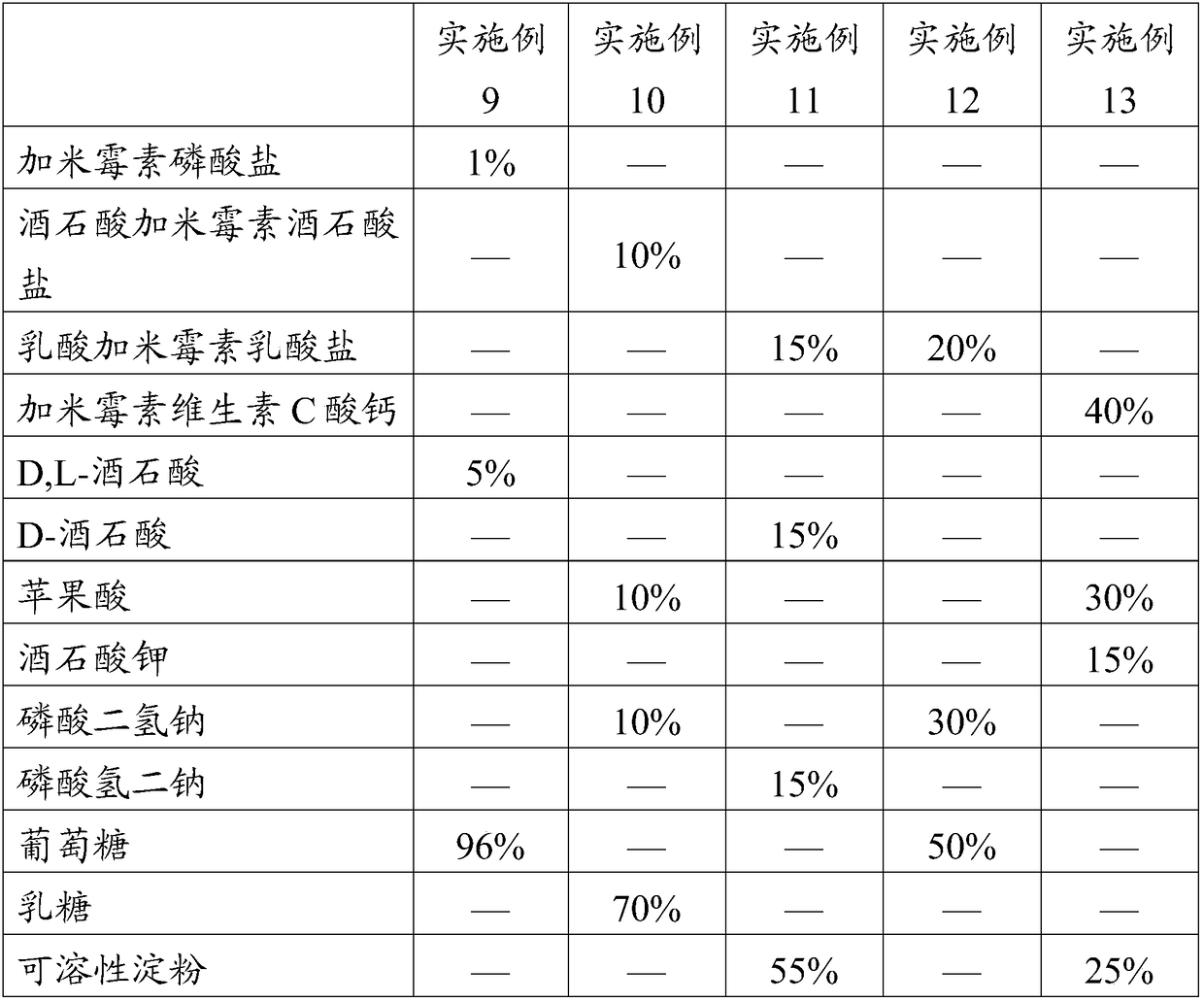
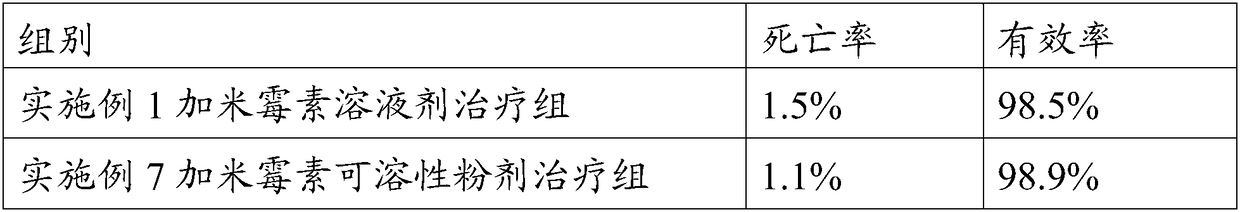
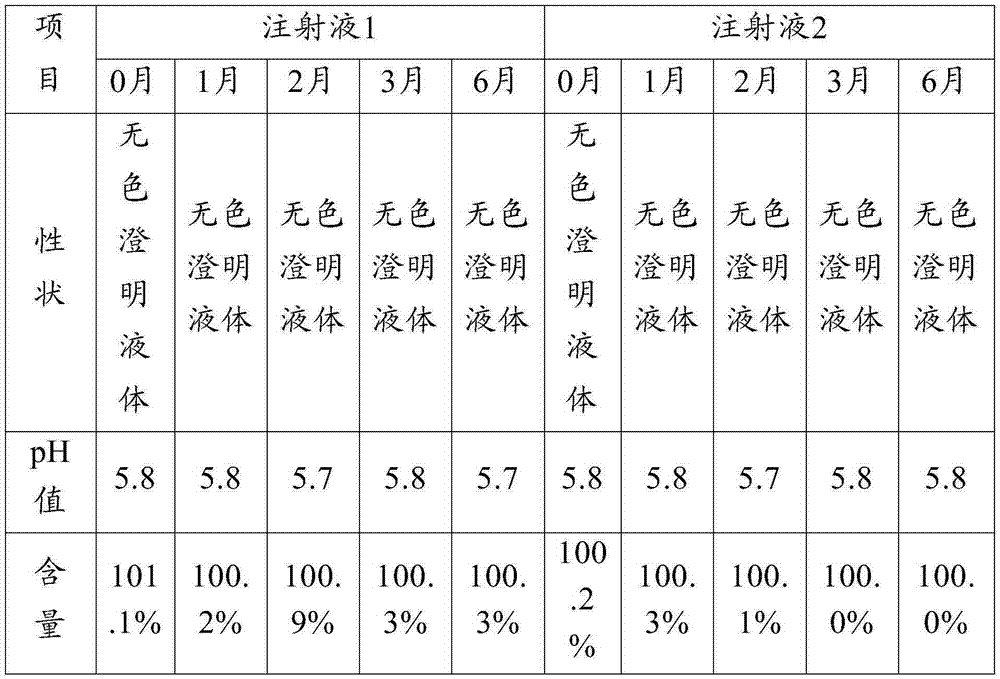
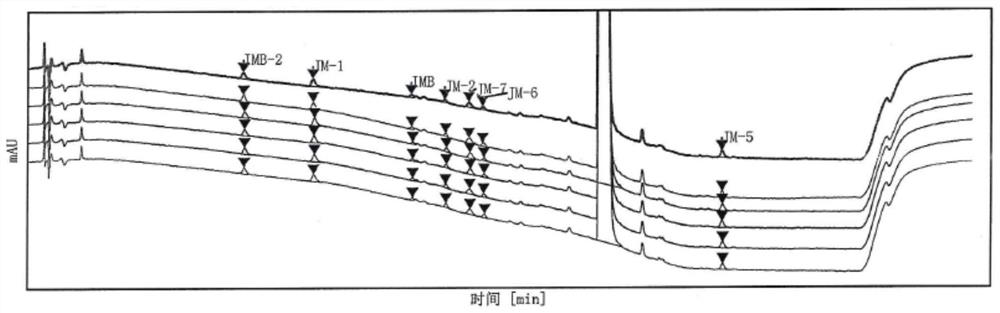
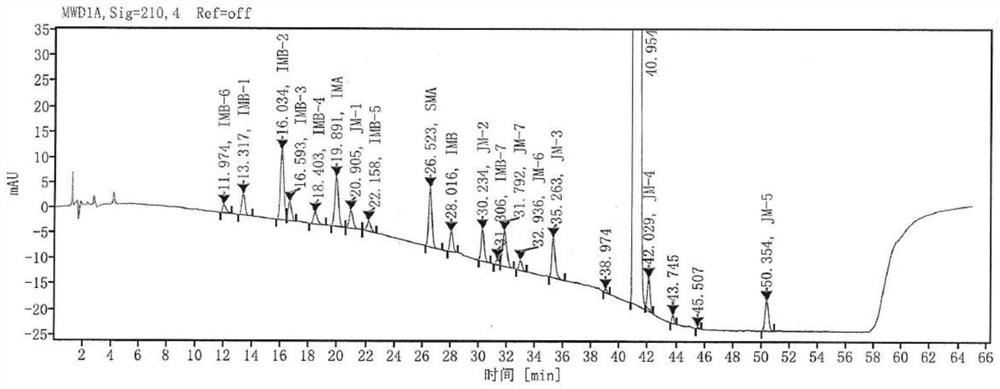
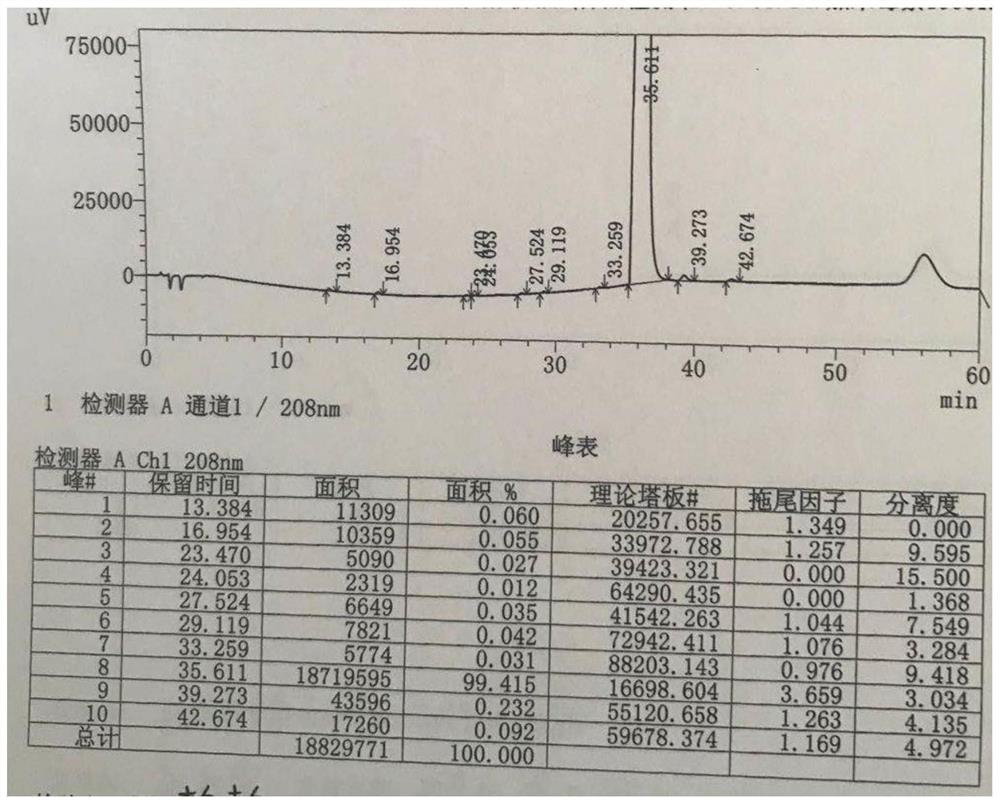
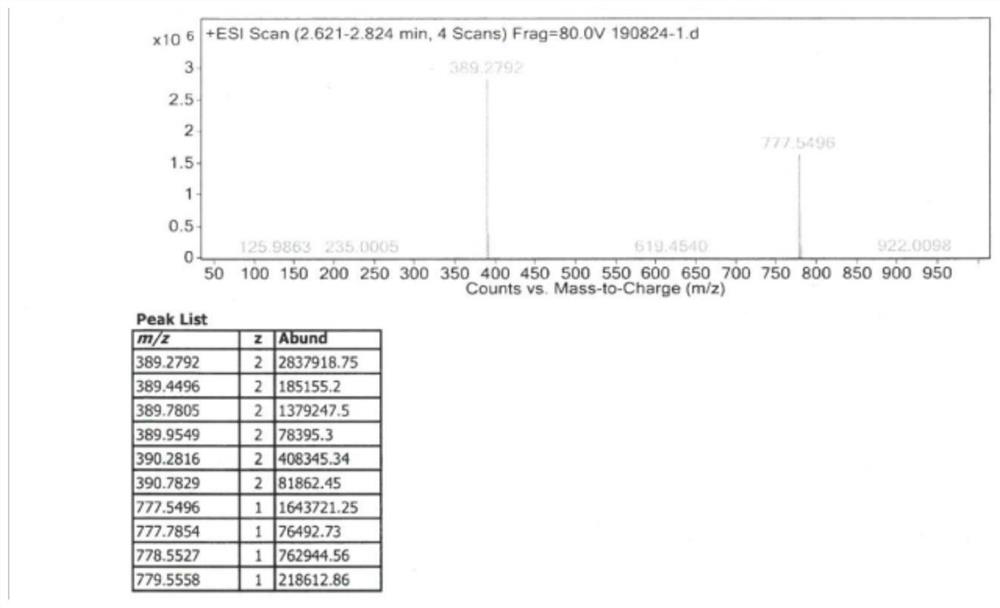
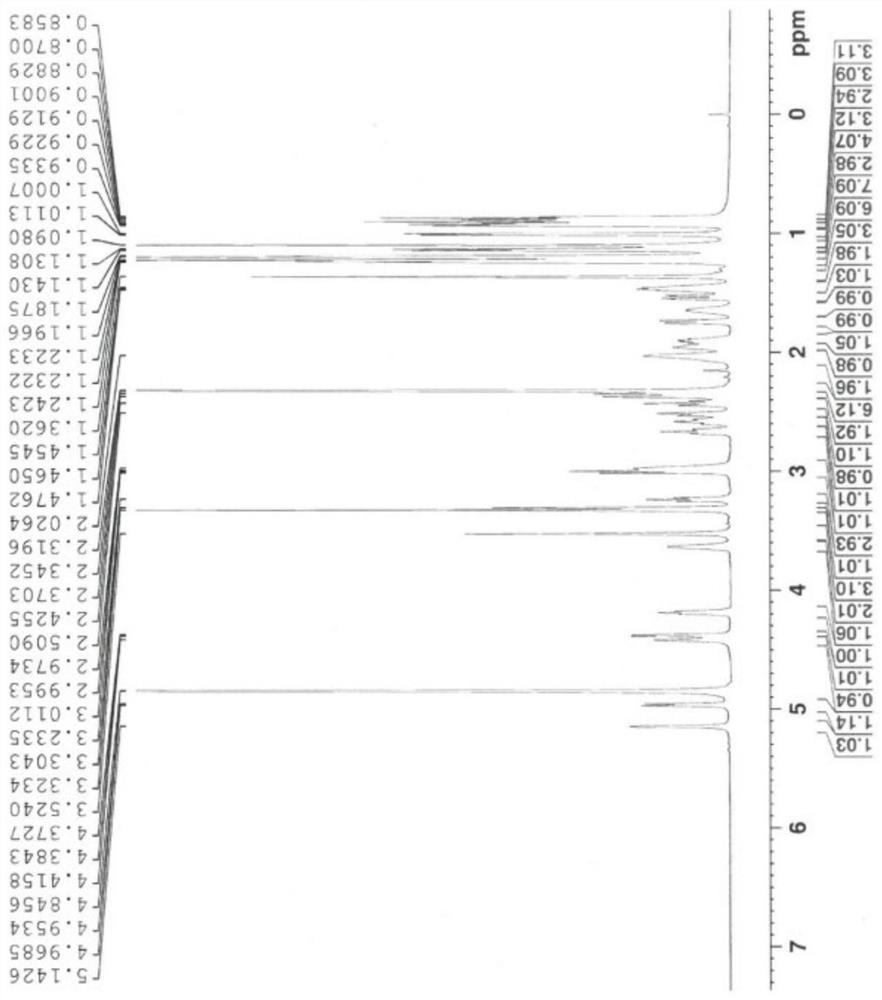
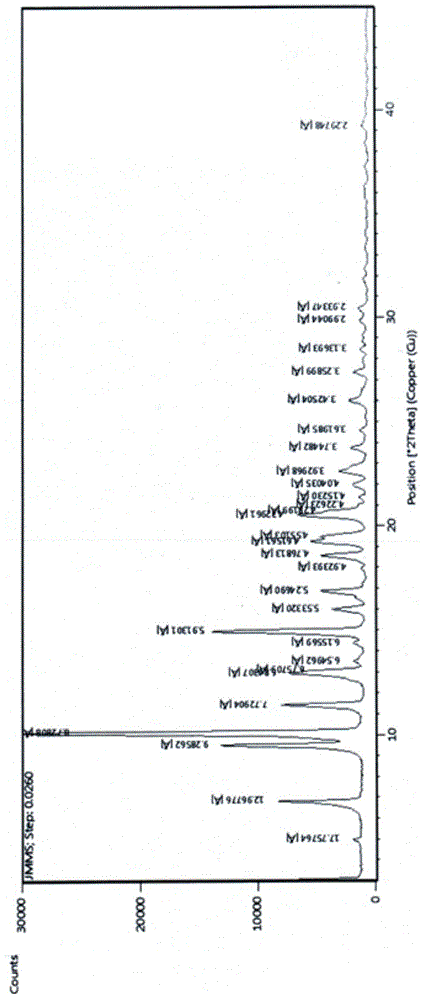
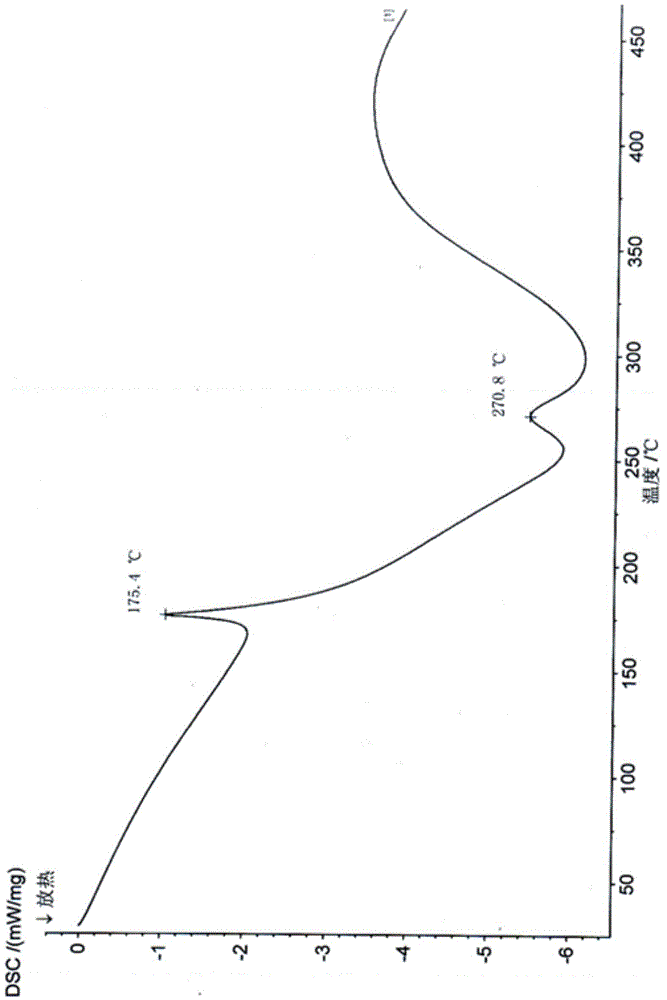
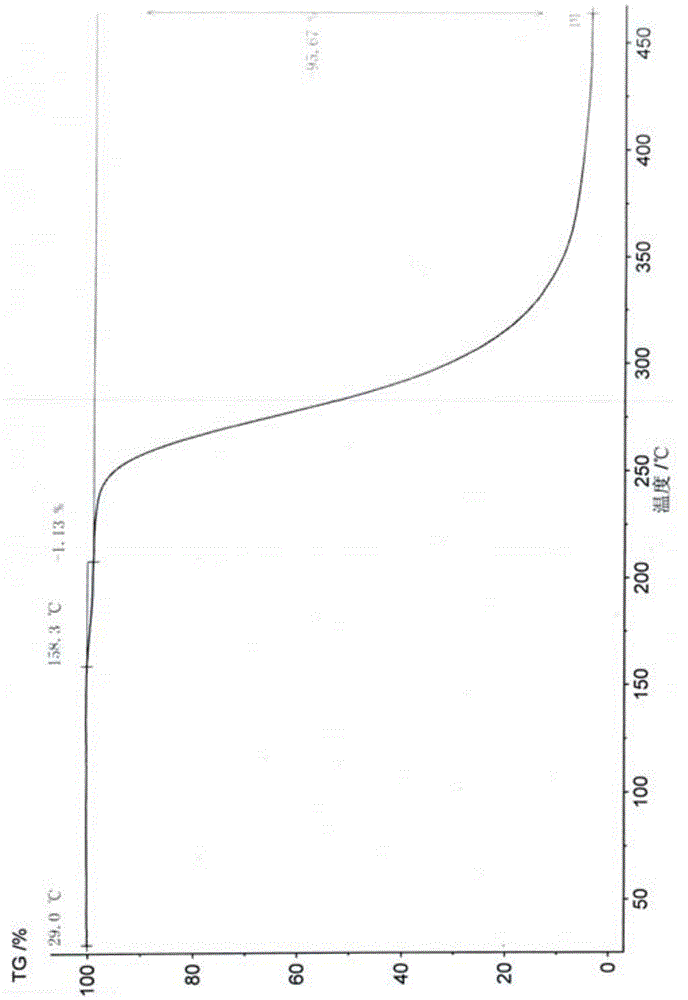
Patents
Literature
41 results about "Gamithromycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gamithromycin injection and preparation method thereof
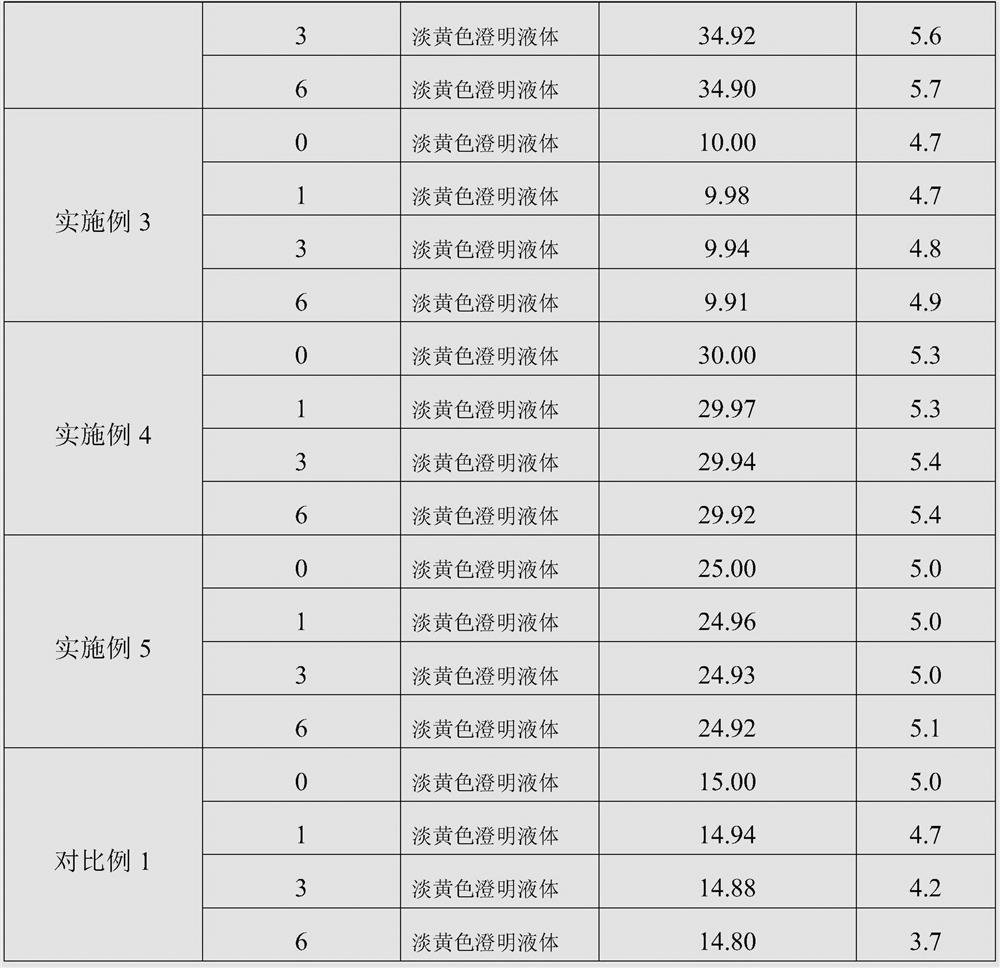
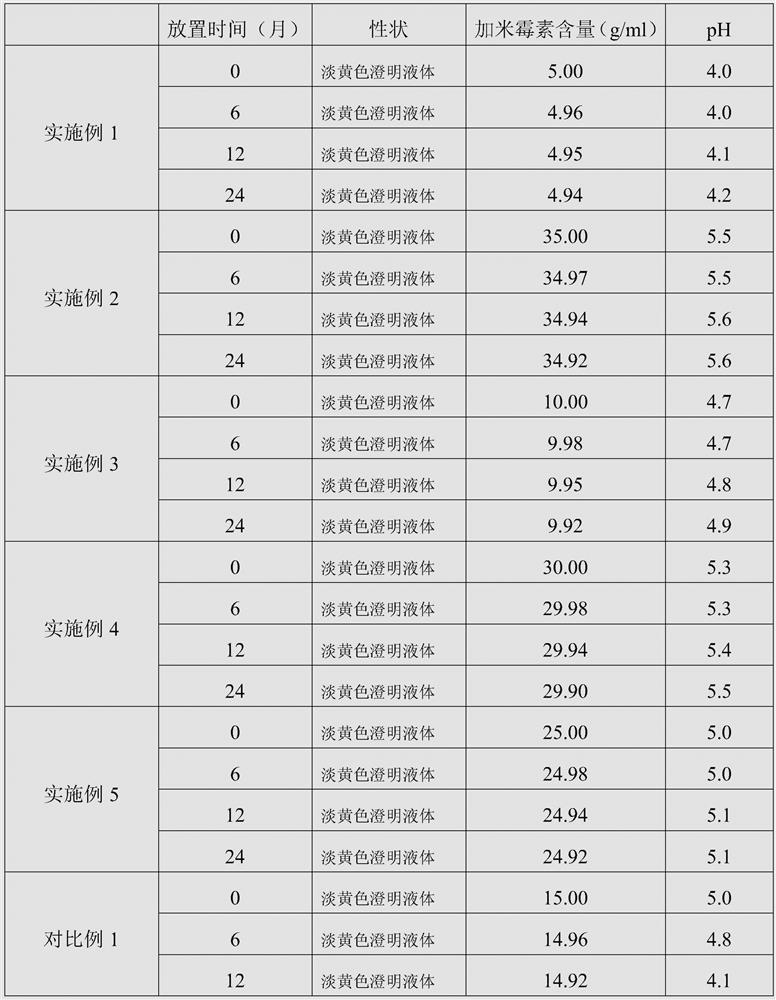
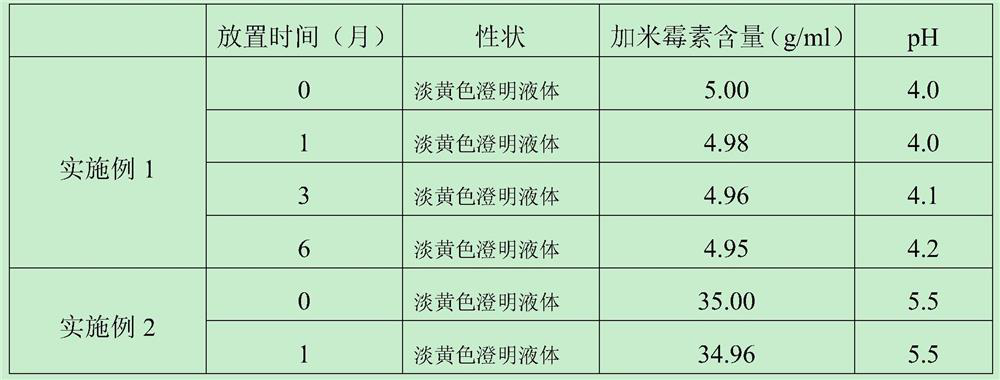
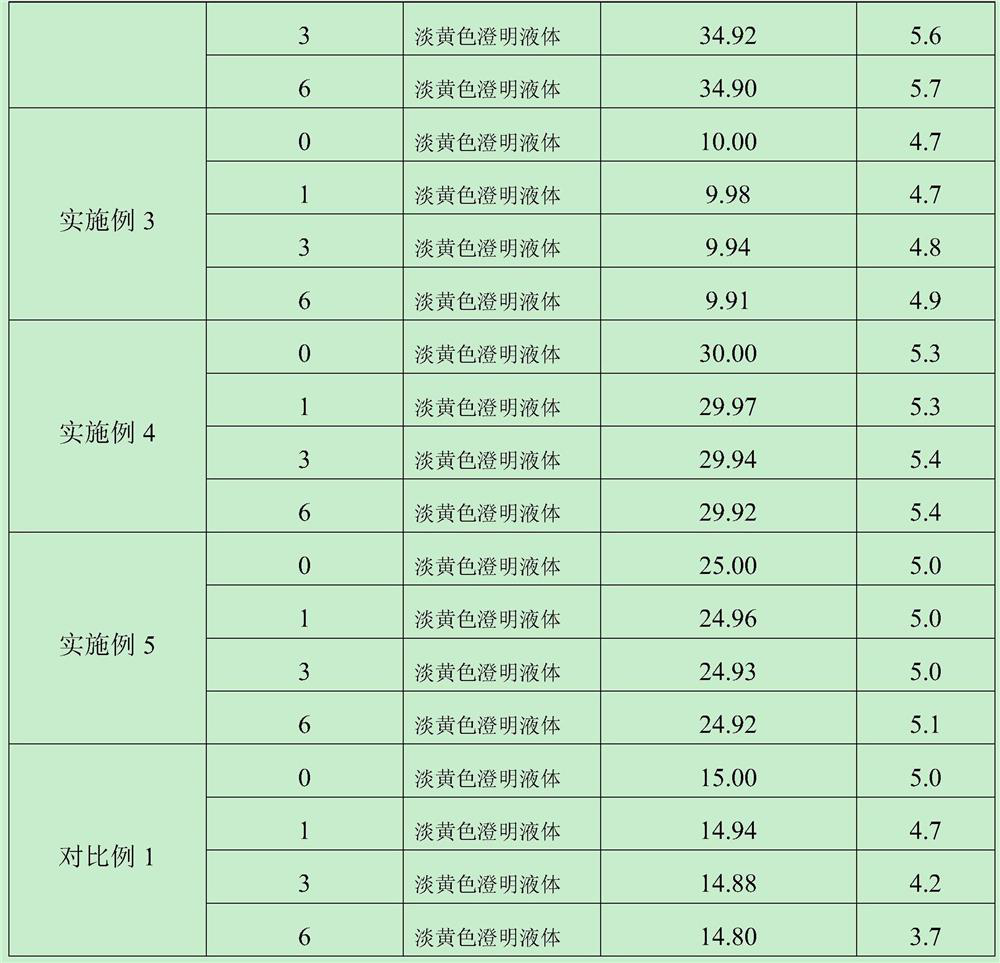
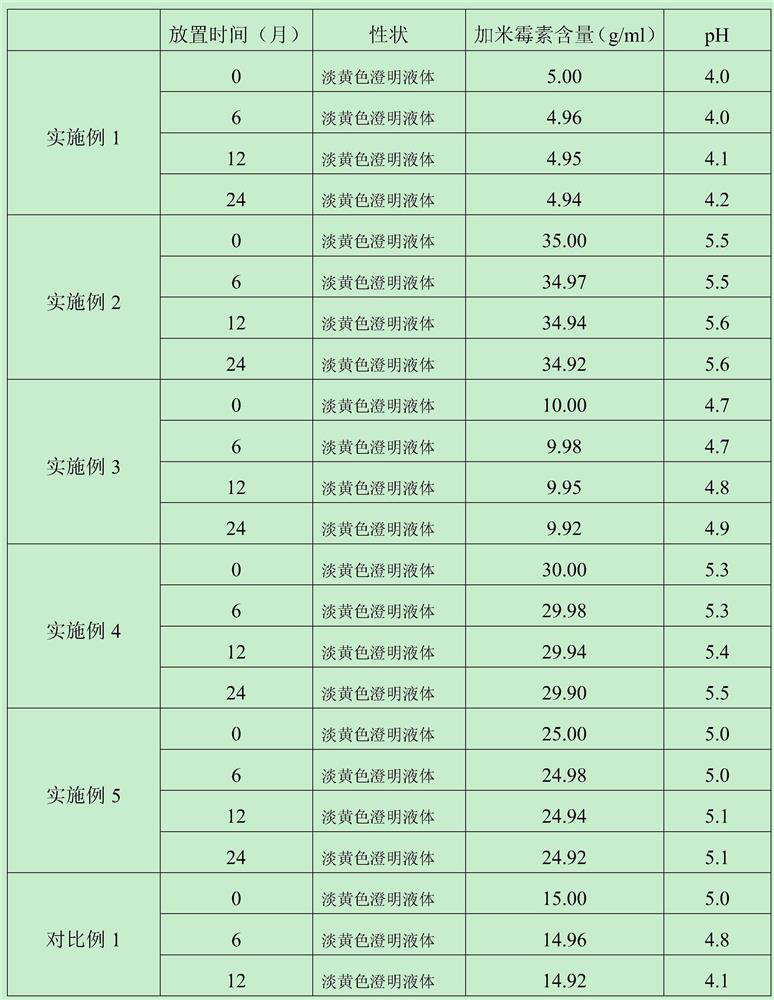
ActiveCN103462884AExpand the scope of clinical applicationStable in natureAntibacterial agentsOrganic active ingredientsGamithromycin Injectable SolutionAntioxidant
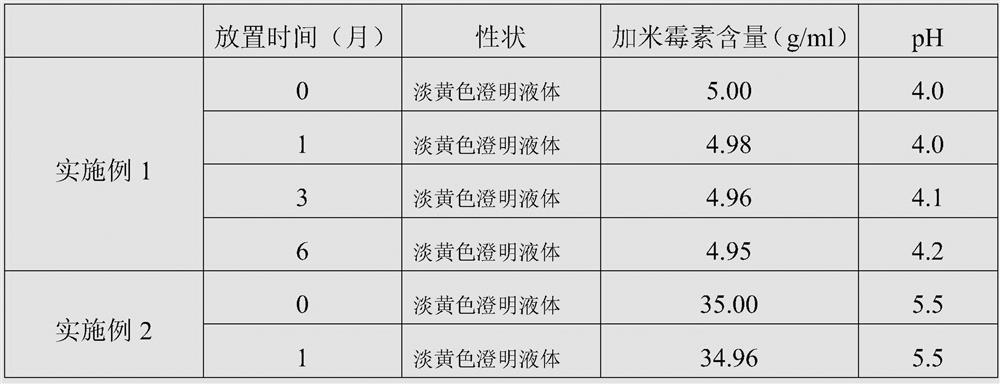
The invention discloses a gamithromycin injection and a preparation method thereof. The gamithromycin injection is prepared from the components including gamithromycin, a cosolvent, an antioxidant and water for injection, and is characterized by comprising 5-35 g of gamithromycin, 1-15 ml of the cosolvent, 0.1-0.5 g of the antioxidant and the balance of water for injection in every 100 ml injection. The preparation method comprises the steps as follows: dissolving the cosolvent in the water for injection, continuously adding gamithromycin and stirring to enable the gamithromycin to be dissolved, then adding the antioxidant to adjust pH to be 4.0-6.0, filtering and sterilizing, so that the gamithromycin injection is obtained. The gamithromycin injection is stable in properties, fast in absorption, remarkable in effect and simple in the preparation method, has less irritation to an injection part, effectively solves the problem that the gamithromycin is indissolvable in water, and meets the requirement of mass industrial production.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Method for detecting content of gamithromycin
InactiveCN103713080AAccurate methodSimple methodComponent separationDipotassium phosphateLength wave
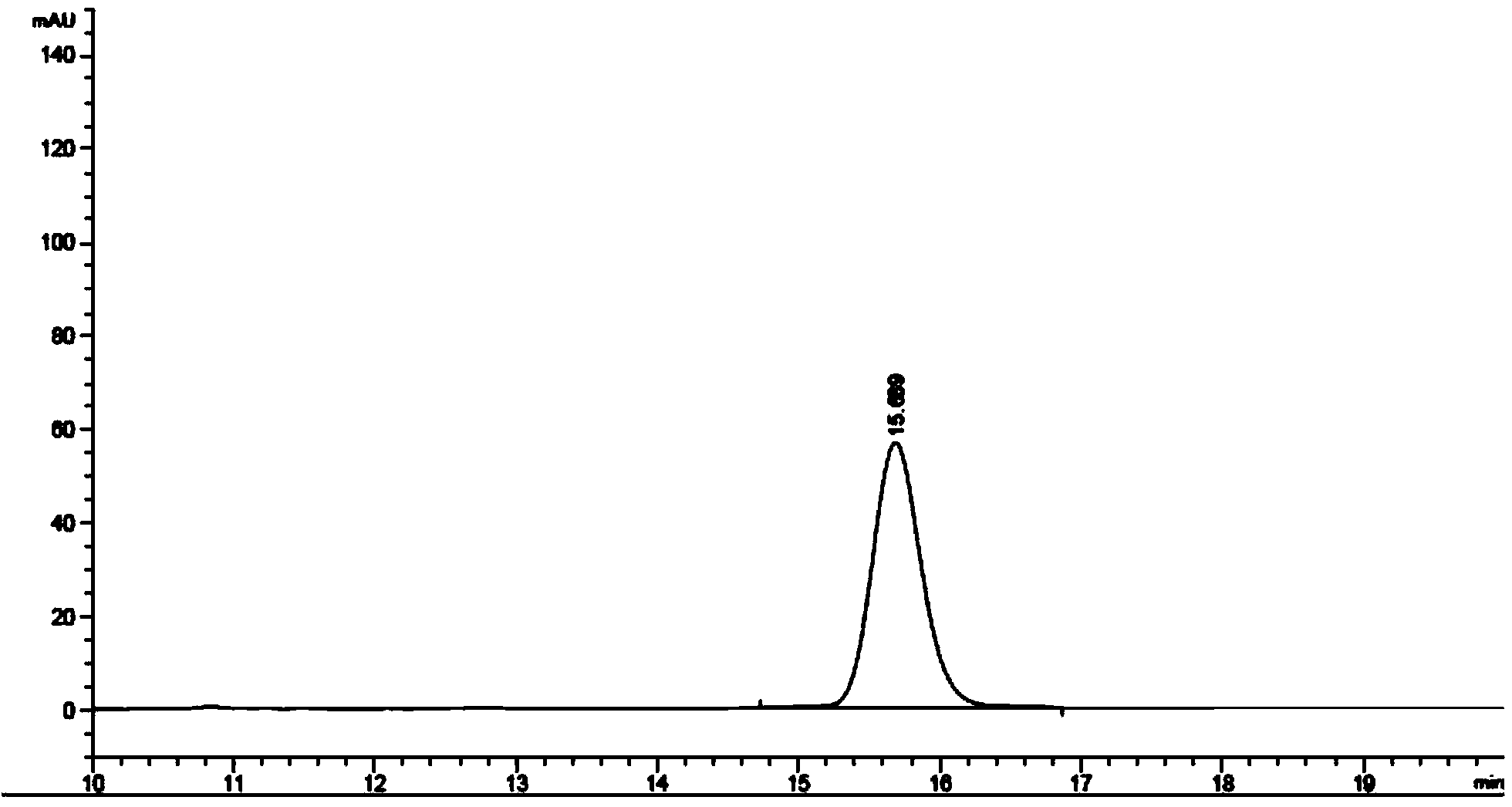
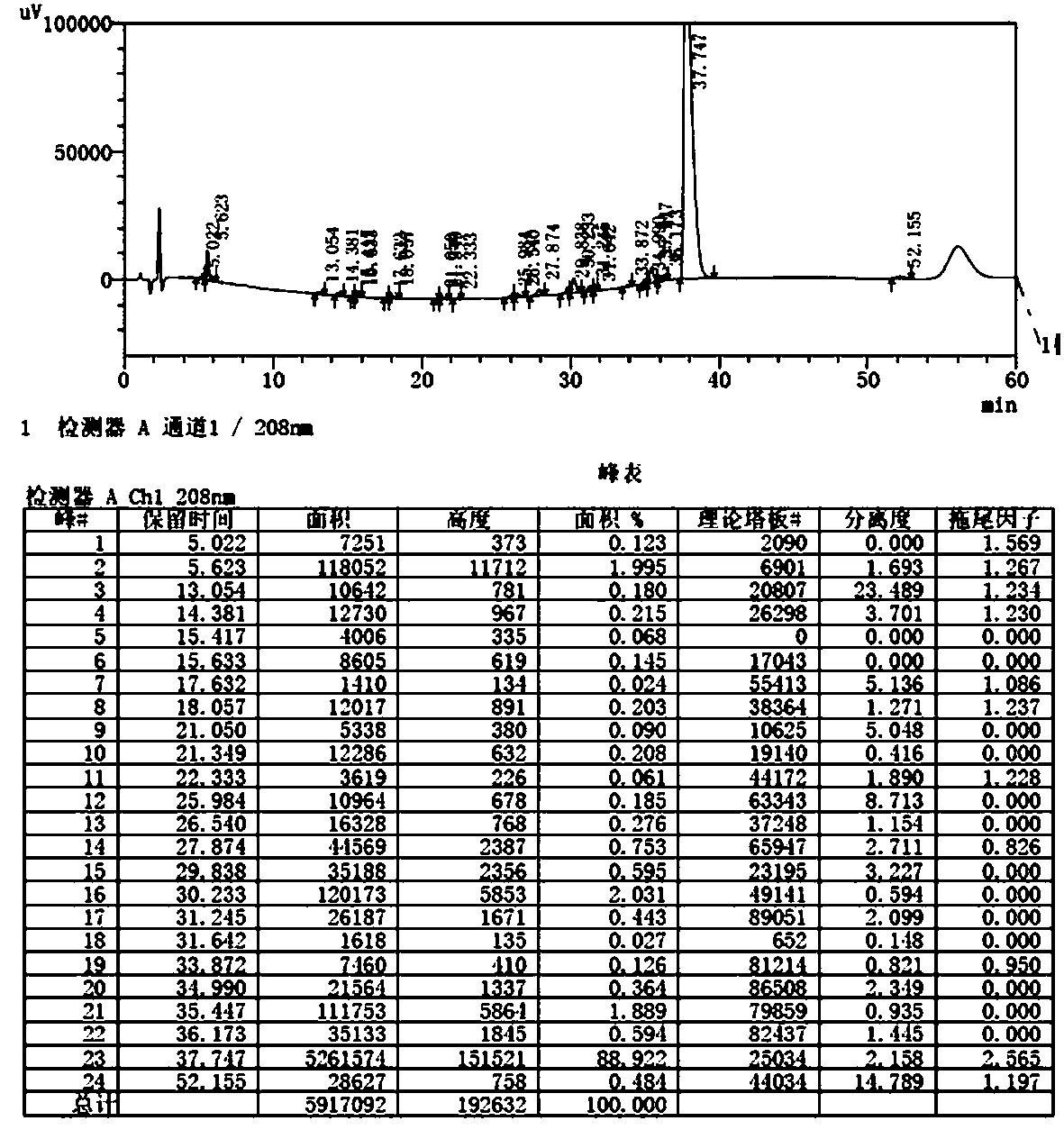
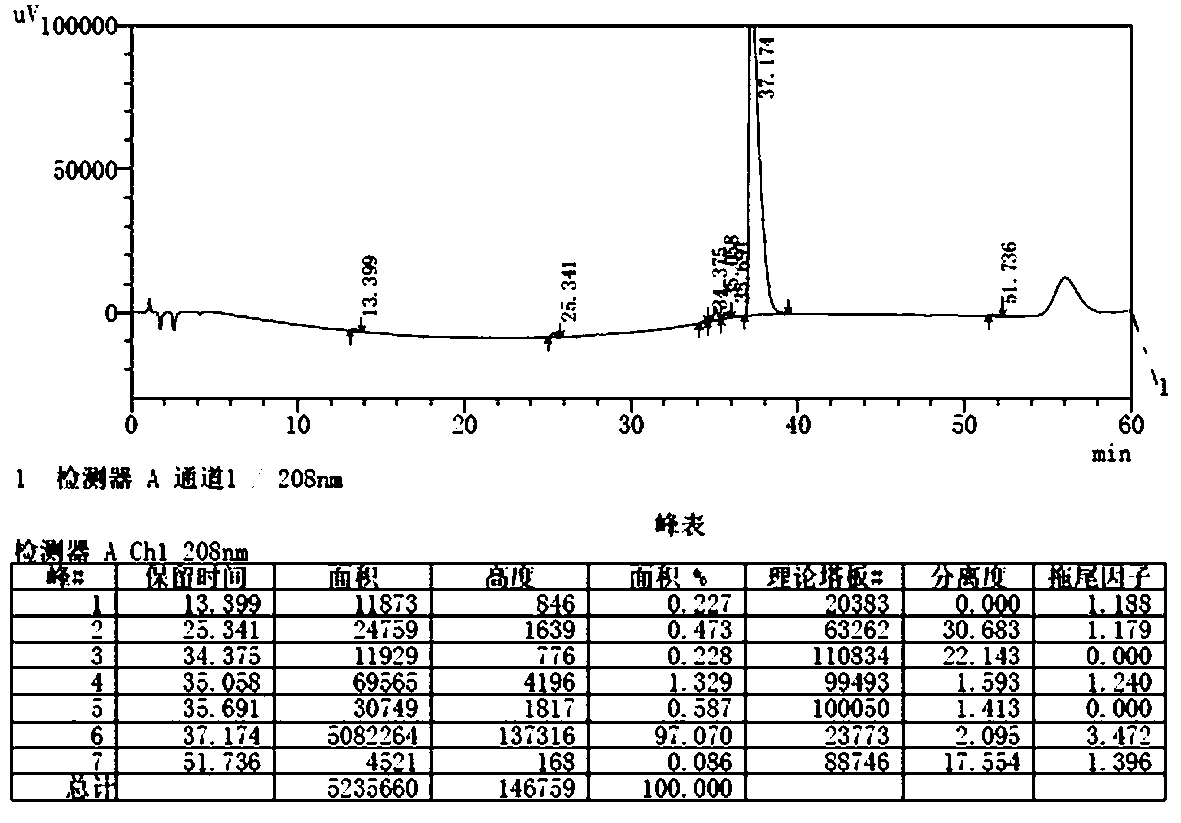
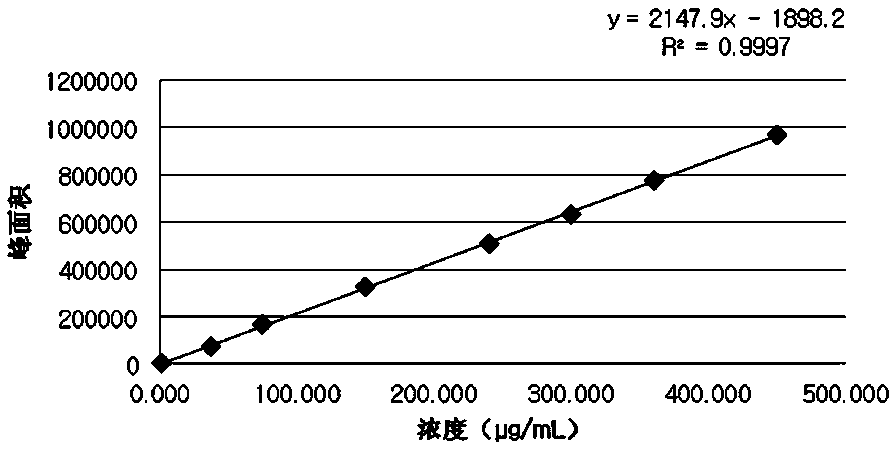
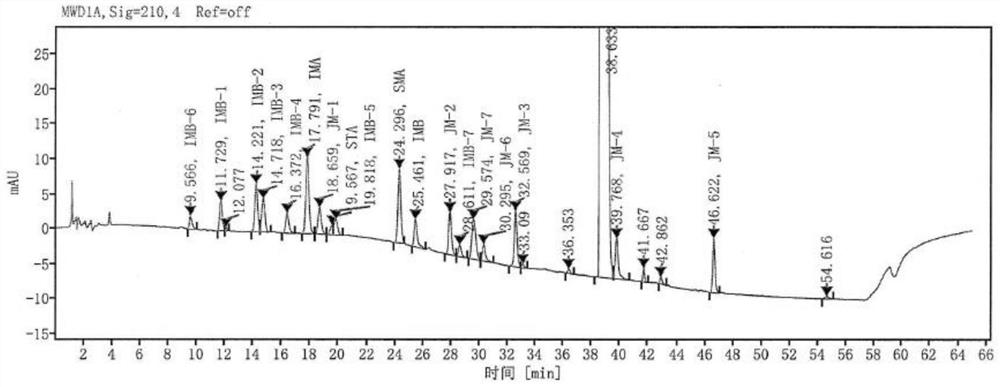
The invention provides a method for detecting the content of gamithromycin. The method comprises the steps: dissolving a gamithromycin sample to be detected by a flowing phase, and performing liquid phase chromatogram quantitative analysis by using a DAD (Dioxide Array Detector) detector adopting C8 and C18 chromatographic columns, wherein chromatographic conditions are as follows: the flowing phase is as follows: a weight ratio of acetonitrile to 0.025mol / ml dipotassium phosphate solution (PH8-8.5) is 70:30, a flow velocity is 1.00-2.00ml / min, and a detection wavelength is 210-230nm. The method is accurate and simple, and is good in reproducibility and high in sensitivity.
Owner:挑战(天津)动物药业有限公司
Gamithromycin composition lyophilized powder for injection and preparation method
ActiveCN103494780ALess irritatingImprove stabilityAntibacterial agentsPowder deliverySuccinic acidBULK ACTIVE INGREDIENT
The invention discloses a gamithromycin composition lyophilized powder for injection. The gamithromycin composition lyophilized powder contains gamithromycin serving as an active ingredient and pharmaceutical excipients, the pharmaceutical excipients are at least one of citric acid, L-malic acid, tartaric acid and succinic acid, the weight ratio of the active ingredient and the pharmaceutical excipients is 1:0.27-0.65, and the pH value of a solution is adjusted to be 5.5-6.0 by using sodium hydroxide before lyophilizing. The invention further provides a preparation method of the gamithromycin composition lyophilized powder for injection. By the method, sterile gamithromycin composition lyophilized powder can be prepared; during clinic use, the gamithromycin composition lyophilized powder is dissolved in sterile normal saline, the solution is small in viscosity, easy for suction and easy for injection, and the gamithromycin composition lyophilized powder is simple and convenient in clinic use and small in irritancy to animals. Consequently, stability, safety and convenience in use of gamithromycin for injection are improved.
Owner:QILU ANIMAL HEALTH PROD
Gamithromycin injection and preparation method thereof
ActiveCN112386572AGood solubilization effectAntioxidantAntibacterial agentsOrganic active ingredientsGamithromycin Injectable SolutionVitamin C
The invention discloses a gamithromycin injection and a preparation method thereof. The gamithromycin injection is composed of gamithromycin, vitamin C and water for injection. Every 100 ml of the gamithromycin injection comprises 5-35 g of gamithromycin, 1-10 g of vitamin C and the balance water for injection. In the gamithromycin injection, the vitamin C is used to increase a dissolving speed and a solubility of the gamithromycin raw medicine, has a very good solubilizing effect, also has an antioxidant effect, and can improve stability of the product. The gamithromycin injection is a weaklyacidic water-phase injection, can be matched with most of medicines for use, greatly increases long-term storage stability of gamithromycin, has small irritation to an injection part, quick absorption, a remarkable effect, high safety and simple prescription, is convenient for controlling quality of raw materials and auxiliary materials, saves cost, and is suitable for industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Content detection method of gamithromycin
ActiveCN104535693AStable baselineLow absorption wavelengthComponent separationChemical structureHplc method
The invention provides a content detection method of gamithromycin. According to the technical scheme, the content of gamithromycin is detected by virtue of a HPLC method; an evaporative light-scattering detector is utilized, is high in sensitivity and is not sensitive to temperature variation, and a base line is relatively stable; the response of the evaporative light-scattering detector does not depend on the optical property of a sample, is not influenced by functional groups, is not interfered by a mobile phase and is in direct proportion to the quality of the sample, and specific chemical structures of detected main components are not required; the gamithromycin is relatively low in absorption wavelength and is absorbed at a tail end of an ultraviolet ray; therefore, compared with an ultraviolet detector, the evaporative light-scattering detector has great superiority in the detection of gamithromycin. Besides, selected chromatographic columns and chromatographic conditions are designed tightly around the characteristic of the evaporative light-scattering detector, a relatively good separation effect is integrally realized; after the method is verified by virtue of methods such as an accuracy experiment, a linear relation experiment and a sampling recycling experiment, results show that the accuracy is high, the detection range is wide, and the sensitivity is relatively good.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
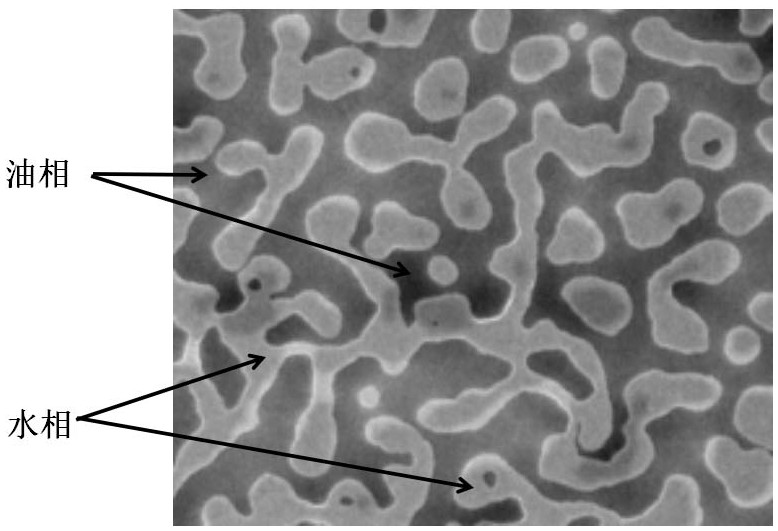
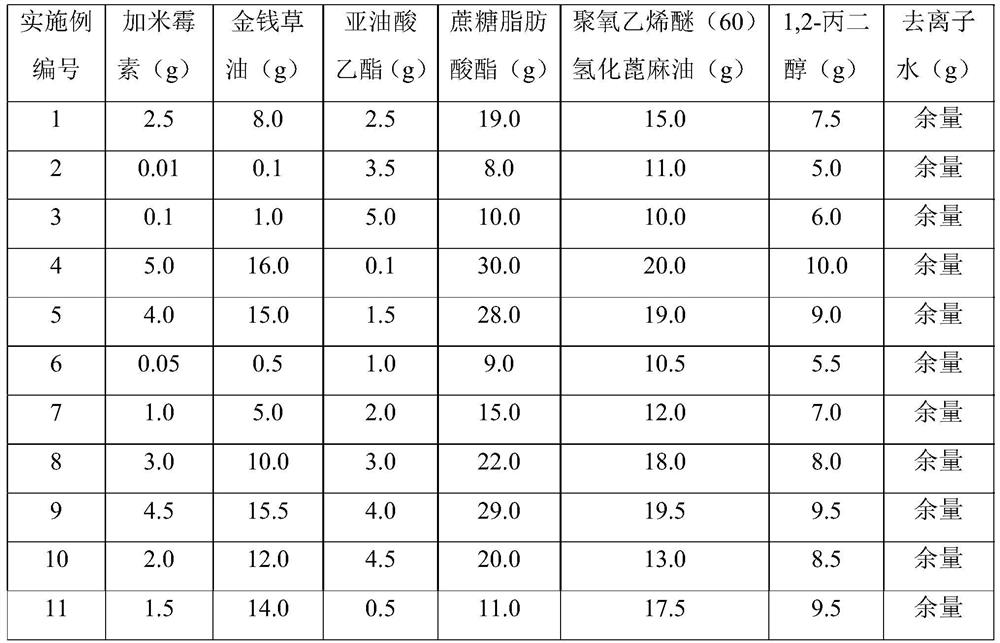
Gamithromycin emulsion, preparation method thereof and application of gamithromycin emulsion in prevention and treatment of porcine ileitis
ActiveCN112972379APrevention and treatment of ileitisConducive to clinical promotion and applicationAntibacterial agentsOrganic active ingredientsSucroseToxicology
The invention relates to a gamithromycin emulsion. Each 100g of the gamithromycin emulsion comprises the following components by weight of 0.01 to 5.0 g of gamithromycin, 0.1 to 16.0 g of lysimachia christinae hance oil, 0.1 to 5.0 g of ethyl linoleate, 8.0 to 30.0 g of sucrose fatty acid ester, 10.0 to 20.0 g of polyoxyethylene ether (60) hydrogenated castor oil, 5.0 to 10.0 g of 1, 2-propylene glycol and the balance of deionized water. Meanwhile, the invention further discloses a preparation method, the process operability is high, and production conversion is facilitated. The emulsion disclosed by the invention is in an oil-water mutual wrapping type, the prepared product can be infinitely diluted with water and can also be infinitely diluted with oil, after being diluted with water, the emulsion can meet the drinking water administration requirement and is convenient to use, and after being diluted with oil, the emulsion can be prepared into a slow-release emulsion, so that a long-acting antibacterial effect is achieved, and the administration frequency is reduced. The emulsion disclosed by the invention can be used for preventing and treating ileitis diseases caused by pig Lawsonia intracellularis infection, has an effect obviously better than that of the prior art, and has a wide market application prospect.
Owner:项朝荣
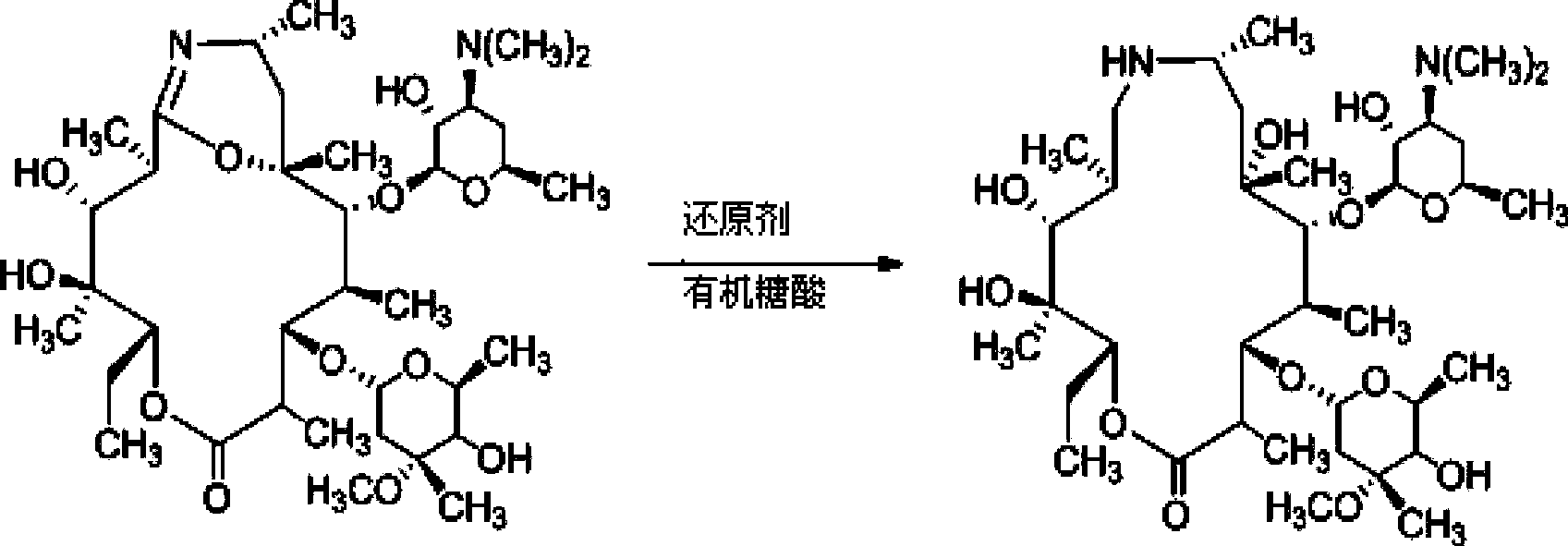
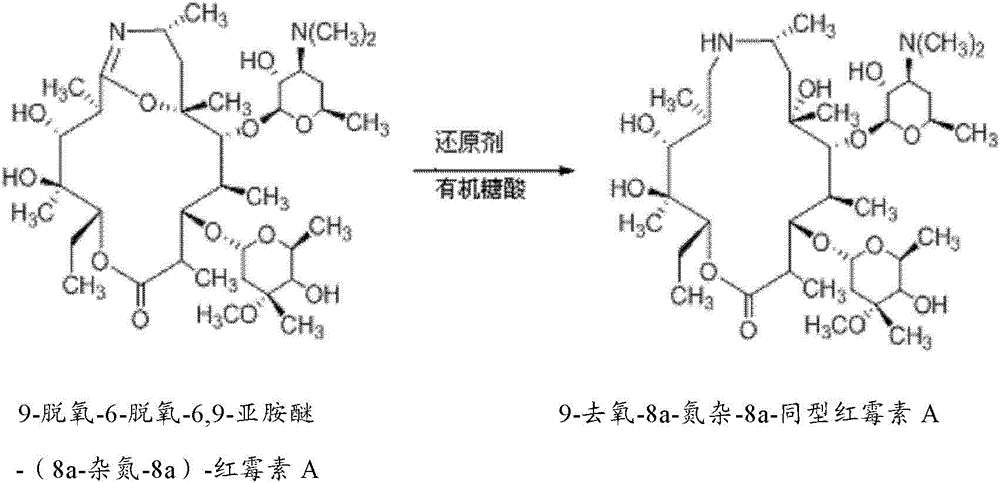
Preparation method of Gamithromycin intermediate
ActiveCN103833807AHigh yieldReduce manufacturing costSugar derivativesSugar derivatives preparationPotassium borohydrideOxygen
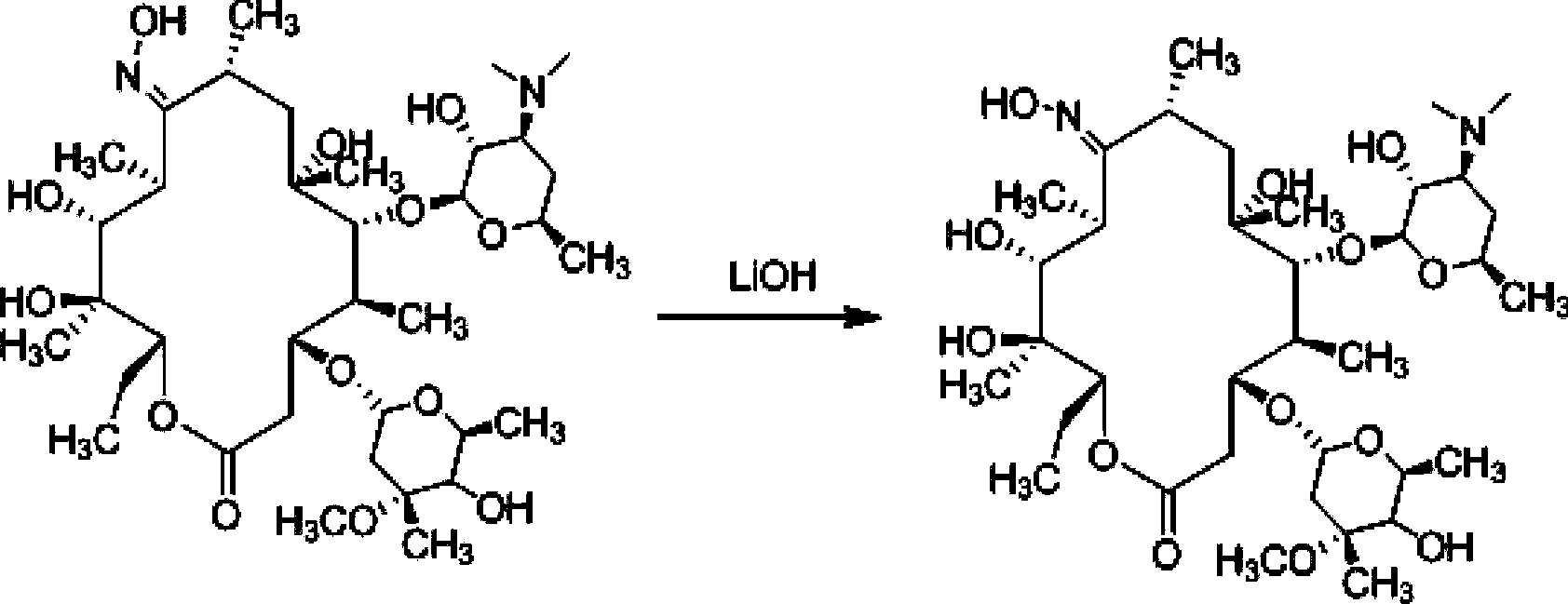
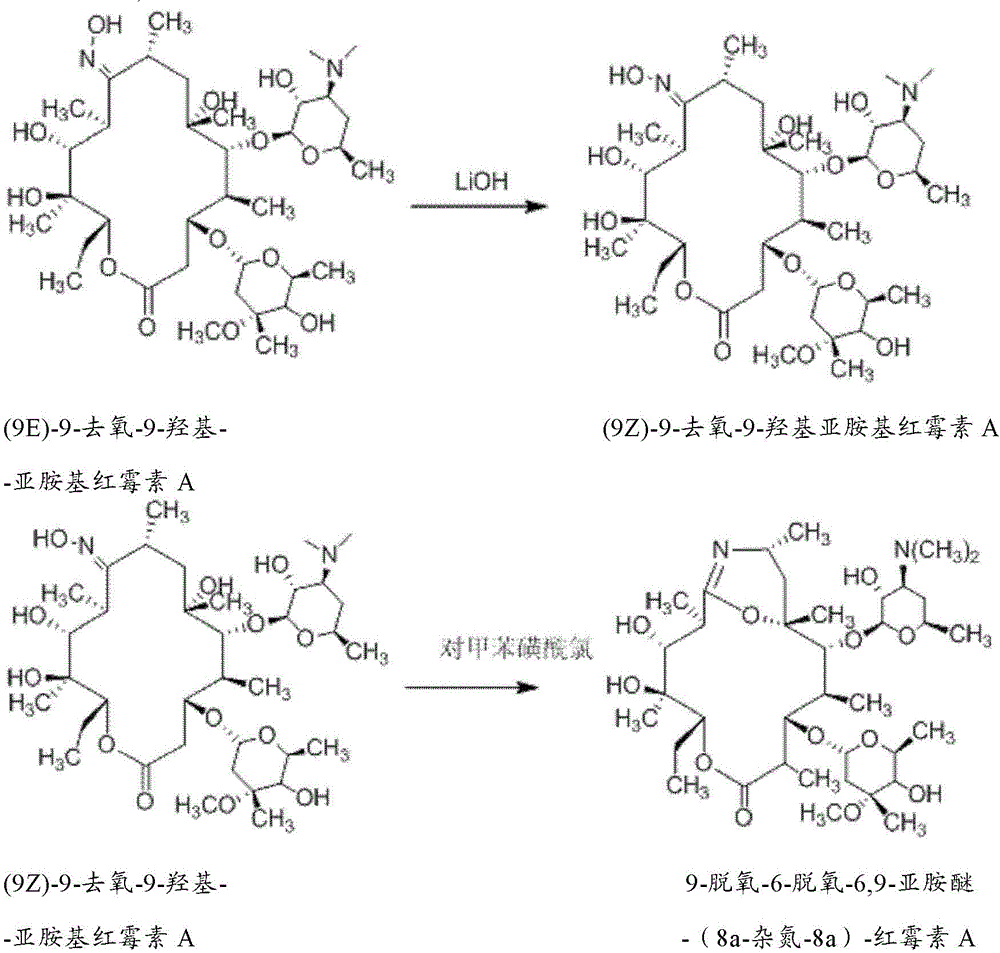
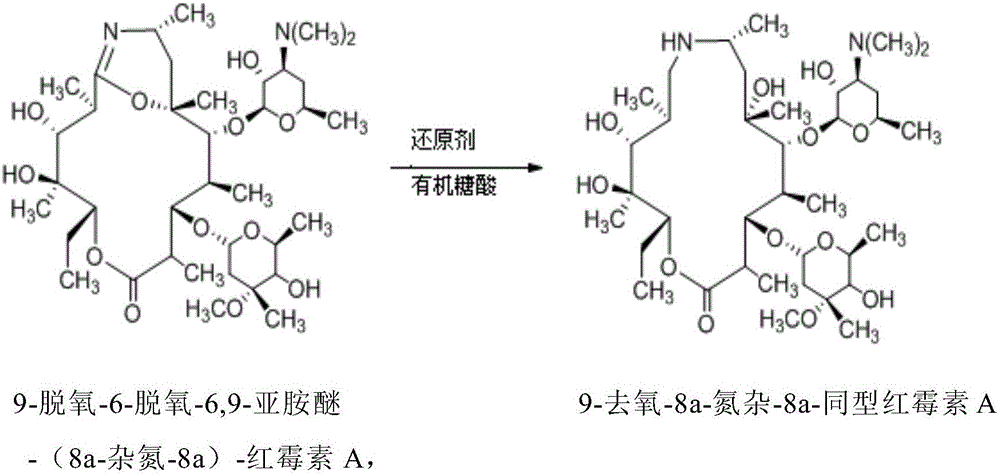
The invention relates to a preparation method of a Gamithromycin intermediate. The method includes: taking 9-deoxy-6-deoxy-6, 9-imino ether-(8a-aza-8a)-erythromycin A as the raw material, dissolving it in an organic solvent, adjusting the pH to 3-6 with acid, adopting potassium borohydride or sodium borohydride as a reducing agent to conduct reduction reaction; and then adding organic sugar acid to undergo hydrolysis reaction to obtain 9-deoxy-8a-aza-8a-homoerythromycin A. As the organic sugar acid contains hydroxyl and is conducive to hydrolysis of a Gamithromycin borate intermediate, the acid degradation product is reduced, and hydrolysis of 9-deoxy-6-deoxy-6, 9-imino ether-(8a-aza-8a)-erythromycin A can be effectively reduced, the yield of the target product is enhanced, and the preparation cost of the Gamithromycin intermediate 9-deoxy-8a-aza-8a-homoerythromycin A can be significantly reduced. Thus, the preparation method is convenient for industrial production.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Gamithromycin monocrystalline type substance and preparation method thereof
ActiveCN104628797AImprove stabilityImprove bioavailabilitySugar derivativesSugar derivatives preparationSpace groupSingle crystal
The invention relates to a gamithromycin monocrystalline type substance and a preparation method thereof. The gamithromycin monocrystalline type substance is a monoclinic system with a C2 space group, and has chirality. The preparation method comprises the following specific steps: (1) putting every 1g of a gamithromycin white solid with purity over 98% into 7-20ml of an organic solvent, heating and dissolving the white solid, wherein the organic solvent is mixed by methanol, alcohol, acetone, ethyl acetate, chloroform, acetonitrile, dichloromethane or tetrahydrofuran and water in any proportion; (2) cooling and standing for placing after the white solid is completely dissolved, separating out a crystal, and filtering to obtain the gamithromycin monocrystalline type substance. The gamithromycin monocrystalline type substance is a single-crystal type compound, is high in stability in the presence of single crystal, and is relatively obvious in bioavailability in comparison with a polycrystal effect. The preparation method of the gamithromycin monocrystalline breaks through the technical bottleneck that technical staff of the field at the existing stage cannot obtain the gamithromycin monocrystalline type substance, is simple and easy, and is suitable for the large-scale industrial production needs.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Gamithromycin related substance and synthesis and separation method thereof
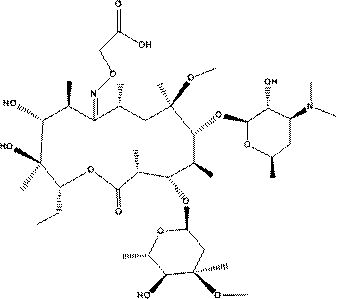
PendingCN111533771ATo satisfy the market's needsHigh puritySugar derivativesSugar derivatives preparationChemical synthesisHydrogen pressure
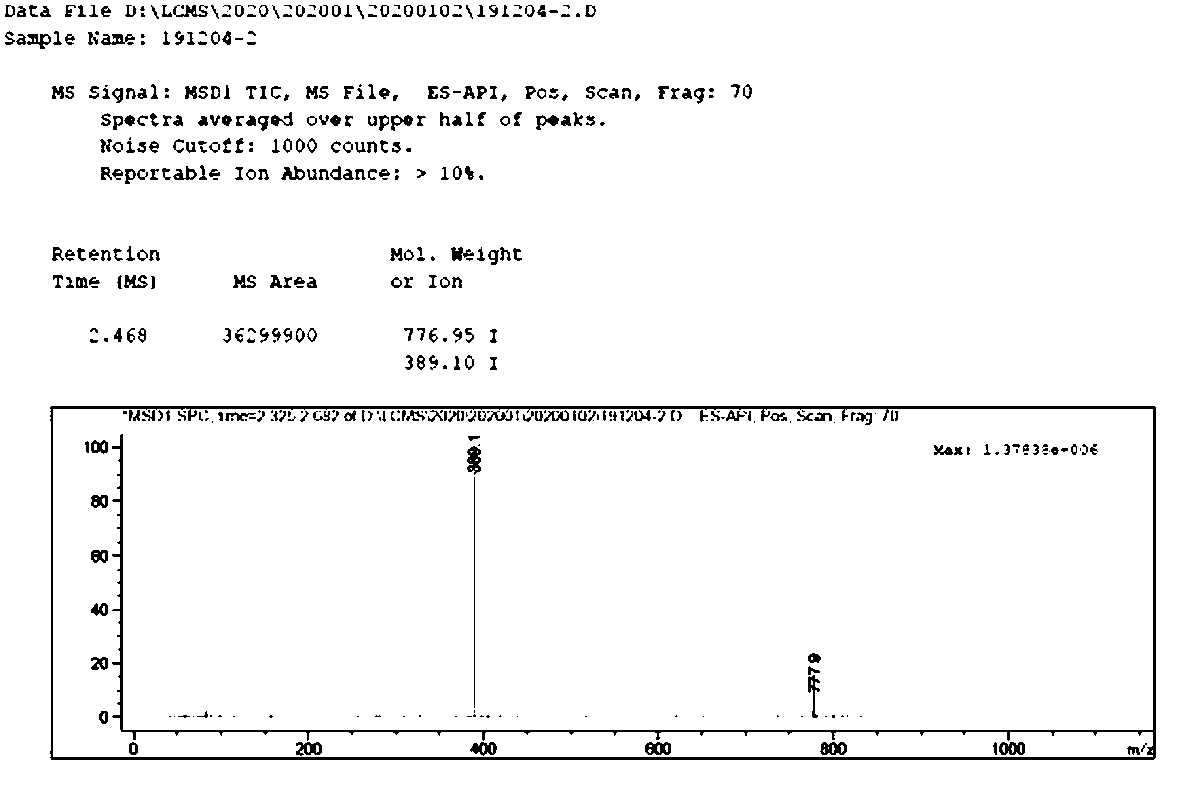
The invention relates to a gamithromycin related substance, and belongs to the field of chemical synthesis. The substance is characterized in that: the gamithromycin related substance is a compound with a structure shown as a formula I or a salt thereof, and the synthesis method takes demethylated azithromycin as a reaction substrate and lower alcohol as a solvent, puts the reaction substrate andthe solvent into a high-pressure reaction kettle, adds propionaldehyde and palladium carbon, keeps hydrogen pressure under a hydrogen protection condition, controls reaction temperature and performs stirring reaction. The product is a necessary product for gamithromycin quality control, the substance prepared by the synthesis and separation method can reach gram grade, and a foundation is laid forgamithromycin quality control and research of unknown impurities.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Gamithromycin injection and preparation method thereof
PendingCN113133968AOvercome the defect of insoluble in waterSolve easy devitrificationAntibacterial agentsOrganic active ingredientsGamithromycin Injectable SolutionAntioxidant
The invention discloses gamithromycin injection and a preparation method thereof, and belongs to the technical field of veterinary pharmaceutical preparations. The gamithromycin injection comprises the following components: gamithromycin, an inclusion agent, an antioxidant, ethanol and water for injection, wherein the inclusion agent is hydroxypropyl-beta-cyclodextrin or sulfobutyl ether-beta-cyclodextrin. According to the injection prepared by carrying out an inclusion reaction on the gamithromycin and the inclusion agent to form an inclusion compound, the defect that the gamithromycin is insoluble in water is overcome, the defects that the gamithromycin injection is easy to crystallize, poor in stability, high in viscosity and serious in animal stress reaction are overcome, and product storage and clinical application are facilitated.
Owner:BAODING JIZHONG PHARMA +2
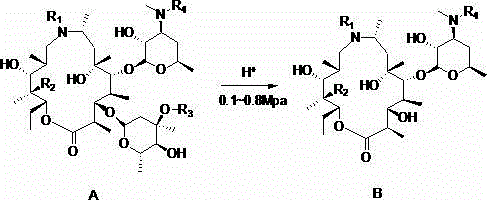
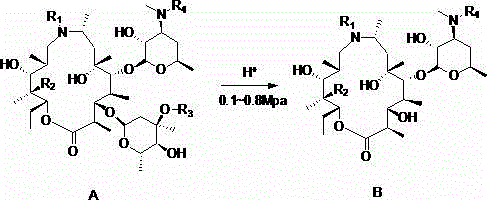
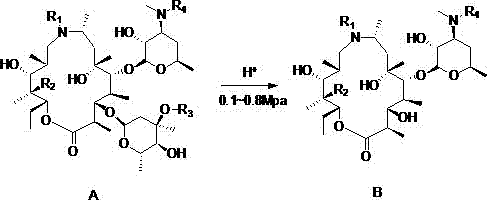
Preparation method of gamithromycin or 13-descladinosylation compound serving as precursor of gamithromycin
ActiveCN104693251AHigh yieldSimple processSugar derivativesSugar derivatives preparationChemical synthesisReaction temperature
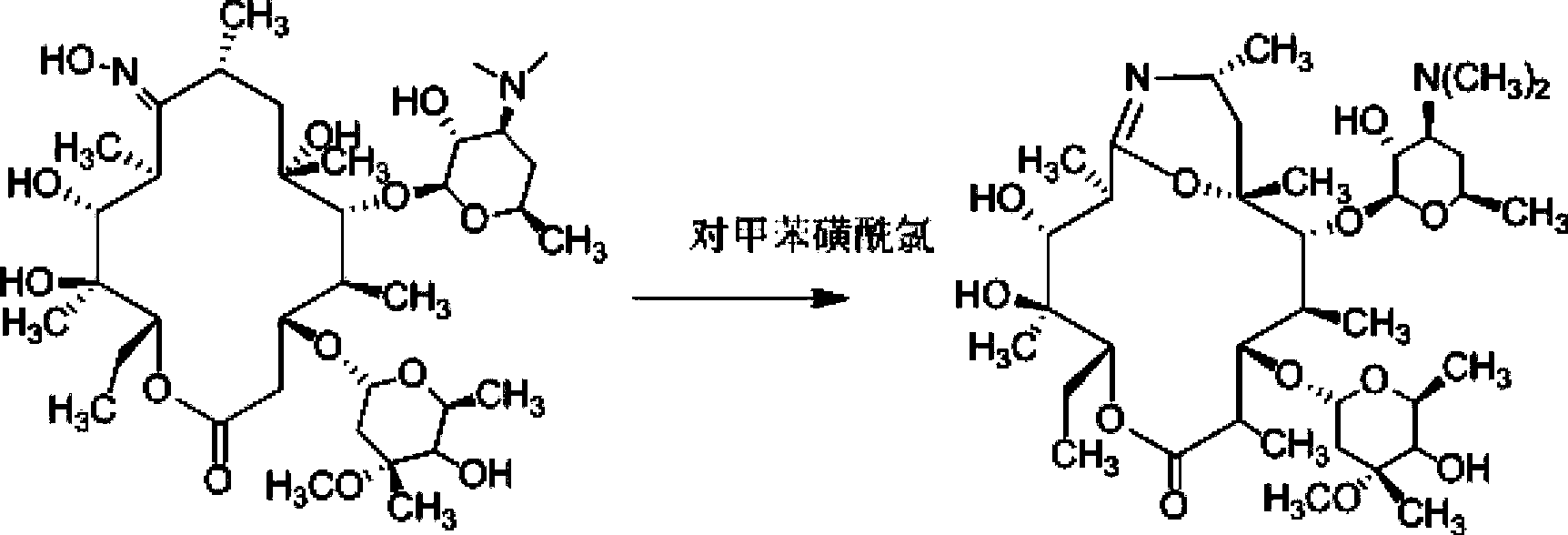
The invention discloses a preparation method of gamithromycin or a 13-descladinosylation compound serving as a precursor of gamithromycin, belonging to the field of chemical synthesis. The method comprises the steps of adding a reaction substrate and a solvent into a high-pressure kettle, wherein the reaction substrate is gamithromycin or the precursor thereof, and the solvent is low-grade amide, low-grade ketone or low-grade alcohol; adding acid, and controlling the pH value of a reaction system at 0.5-5; introducing nitrogen gas to the high-pressure kettle, and keeping the pressure at 0.1-0.8Mpa; and controlling the reaction temperature at 0-35 DEG C and stirring for 1-12 hours. The preparation method is simple in process and high in selectivity, and the yield of gamithromycin or the 13-descladinosylation compound serving as the precursor of gamithromycin is high. Gamithromycin or the precursor thereof contains hydroxyls, ester groups and a plurality of ether bonds; and the reaction substrate of gamithromycin and the precursor thereof in the method disclosed by the invention selectively breaks the ether bonds at the juncture of cladinose and a matrix under an acid condition, but the hydroxyls and the ester groups are unchanged under the reaction condition, so that the selectivity is high.
Owner:QILU SYNVA PHARMA
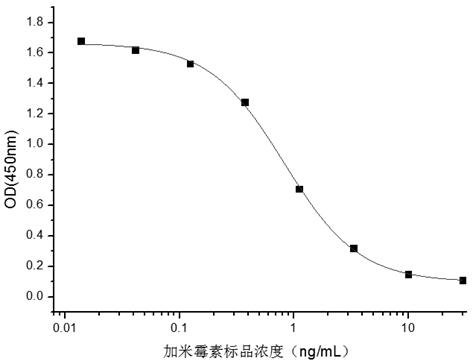
Hybridoma cell strain secreting gamithromycin monoclonal antibody and application of hybridoma cell strain
ActiveCN111778215AHigh detection sensitivityImprove featuresDepsipeptidesTissue cultureImmune profilingImmuno detection
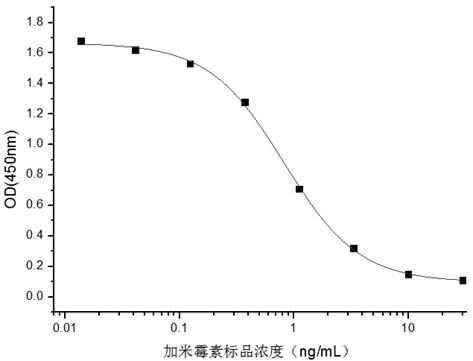
A hybridoma cell strain secreting a gamithromycin monoclonal antibody and application of the hybridoma cell strain, and belongs to the field of food safety immune detection. The hybridoma cell strainABC11 secreting the gamithromycin monoclonal antibody is collected in the China General Microbiological Culture Collection Center (CGMCC) on November 28, 2019 with a collection number of CGMCC No. 19167. The gamithromycin monoclonal antibody secreted by the strain is used for analysis and detection of gamithromycin residues in food safety testing. The gamithromycin monoclonal antibody cell strainobtained in the present invention can be used for immunoassay and detection, and has good detection sensitivity and specificity for gamithromycin (IC50 value is 0.82 ng / mL, the crossover of a gamithromycin analog is less than 10%, and the crossover rate = (IC50 of the gamithromycin / IC50 of the analog) *100%)).
Owner:JIANGNAN UNIV +1
Gamithromycin sustained-release preparation and preparation method thereof
PendingCN112972409AEasy to takeImprove complianceAntibacterial agentsOrganic active ingredientsCelluloseCarboxymethyl cellulose
The invention discloses a gamithromycin sustained-release preparation and a preparation method thereof. The gamithromycin sustained-release preparation comprises the following components in parts by weight: 5-50 parts of gamithromycin, 10-40 parts of a sustained-release framework material and 10-85 parts of auxiliary materials, the sustained-release framework material is prepared from xanthan gum, sodium carboxymethyl cellulose and carrageenan according to the mass ratio of 1: 1: 1, and the auxiliary material is prepared from soluble starch and Jiyi powder according to the mass ratio of 2: 1. The gamithromycin sustained-release preparation prepared by the invention is convenient to take, good in compliance, good in sustained-release effect, good in stability, high in drug loading capacity and high in safety; and the preparation process is convenient and rapid, and is beneficial to industrial large-scale production.
Owner:四川恒通动保生物科技有限公司
A kind of gamimycin injection and preparation method thereof
ActiveCN103462884BExpand the scope of clinical applicationStable in natureAntibacterial agentsOrganic active ingredientsGamithromycin Injectable SolutionAntioxidant
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Method for preparing gamithromycin
ActiveCN106699823AMild responseEasy to scale up productionAntibacterial agentsOrganic active ingredientsChemical synthesisHydrogen
The invention relates to the technical field of chemical synthesis, in particular to a method for preparing gamithromycin. According to the preparation method, 9-deoxy-8a-aza-high erythromycin A and n-propanal are taken as raw materials, hydrogen is taken as a reducing agent, reaction is performed under the catalytic action of Pd / NiO, and gamithromycin is obtained. According to the preparation method, the reaction process is mild, no special hydrogenation device is required, and the production is amplified easily; a catalyst can be recycled many times, the production cost is reduced, and the method is more economical and environmentally friendly and conforms to large-scale industrial production requirements.
Owner:CHINA ANIMAL HUSBANDRY IND
Preparation method of gamithromycin composite solution
InactiveCN113332238AGuaranteed SolubilityLess irritatingAntibacterial agentsOrganic active ingredientsBiotechnologyOrganic solvent
The invention provides a preparation method of a gamithromycin composite solution, and relates to the technical field of veterinary drug antibiotics. The preparation method of the gamithromycin-based composite solution comprises a premix, gamithromycin freeze-dried powder, a cosolvent, a solubilizer, a substrate solvent, a pH value neutralizer and a pH value buffer, and comprises the following steps: precisely weighing 20 parts of the premix, and pouring into a water bath mixing tank for later use; and taking 5-10 parts of the gamithromycin freeze-dried powder, adding the gamithromycin freeze-dried powder into the mixing tank in which the premix is added, then adding 0.8-1.2 parts of the cosolvent, heating in a water bath at the temperature of 60-80 DEG C for 20-30 minutes, and stirring and mixing by using stirring equipment. According to invention, the gamithromycin can be dissolved in the organic solvent in advance by using the premix, and then is mixed with the substrate solvent through the solubilizer, so that the irritation of the composite solution is successfully reduced under the condition of ensuring the solubility of the gamithromycin, and the practicability of the composite solution is improved.
Owner:四川恒通动保生物科技有限公司
Separation, preparation and purification method of gamithromycin related substance
PendingCN110655544ASugar derivativesComponent separationBiochemical engineeringCombinatorial chemistry
The invention discloses a gamithromycin related substance. The gamithromycin related substance is a compound or salt thereof having a structural formula as shown in a formula (I). The gamithromycin related substance can provide guarantee for monitoring the quality of gamithromycin. The invention further discloses a preparation, analysis and separation method of the gamithromycin related substance.The preparation method disclosed by the invention is simple in technology, and can be in large-scale application in the industry.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Method of synthesizing macrolide compounds
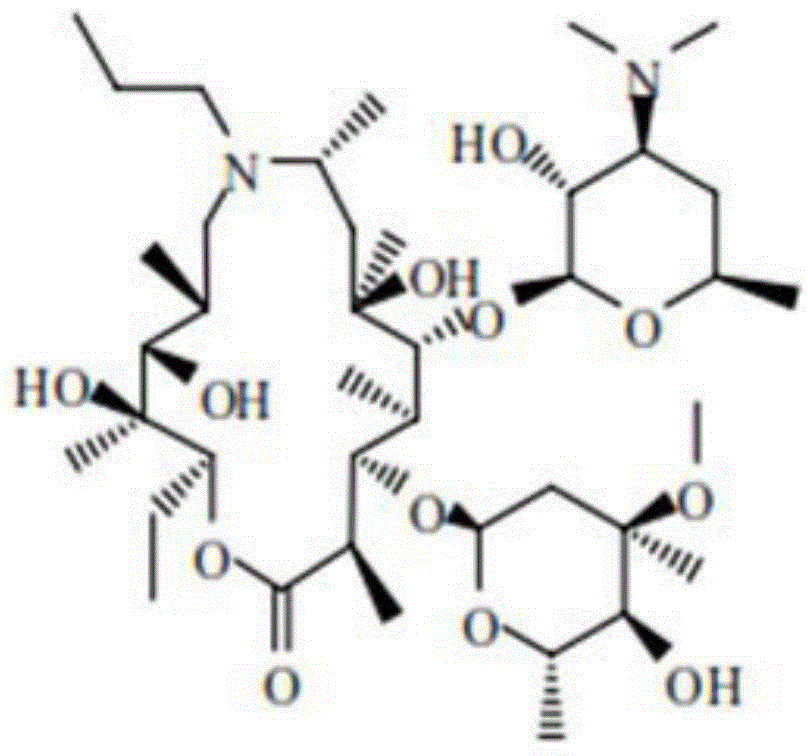
ActiveUS8314218B2Shorten cycle timeReduce the amount requiredAntibacterial agentsSugar derivativesChemical structureChemical reaction
The present invention relates to methods for synthesizing macrolide compounds which are known to have antibacterial activity, and are useful in the therapy of bacterial infections in mammals. More specifically, the invention relates to methods for synthesizing the macrolide antibiotic, gamithromycin utilizing a novel configuration of catalysts, chemical structures, and / or methods. An embodiment of the present invention may include allowing multiple chemical reactions to proceed without the isolation of chemical intermediates. Thus, multiple reactions may occur in one reaction vessel allowing for a considerable decrease in the cycle-time. The present invention also provides a novel method for inhibiting degradation while isolating a structure of a pharmaceutical composition.
Owner:MERIAL INC
Gamithromycin pharmaceutical composition and applications thereof
InactiveCN108567790AHigh antibacterial activityBroad spectrum antibacterialAntibacterial agentsPowder deliveryTreatment effectCure rate
The present invention relates to a gamithromycin composition, which comprises 1-40% of a gamithromycin soluble salt, and other solubilizing components. According to the present invention, the gamithromycin composition can be administered orally so as to reduce the labor intensity of drug application, can reduce antibiotic consumption, and further has high treatment effect, wherein the cure rate ofthe gamithromycin composition is 5-15% higher than the cure rate of the similar products.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
A kind of preparation method of gamitomycin intermediate
ActiveCN103833807BHigh yieldReduce manufacturing costSugar derivativesSugar derivatives preparationOrganic solventPotassium borohydride
The present invention relates to a preparation method of a gamithromycin intermediate, which uses 9-deoxy-6-deoxy-6,9-imino ether-(8a-aza-8a)-erythromycin A as a raw material, Dissolve in an organic solvent, adjust the pH to 3~6 with an acid, and use potassium borohydride or sodium borohydride as a reducing agent to perform a reduction reaction; then add an organic sugar acid to perform a hydrolysis reaction to obtain 9‑deoxy‑8a‑aza ‑8a‑homotype erythromycin A, because the organic sugar acid contains hydroxyl group, which is beneficial to the hydrolysis of gamimycin borate intermediate, the acid degradation products are reduced, and it can effectively reduce 9‑deoxy‑6‑deoxy‑6,9‑sub Amino ether-(8a-aza-8a)-erythromycin A was hydrolyzed, which increased the yield of the target product and significantly reduced the 9-deoxy-8a-aza-8a-homoerythromycin A The preparation cost is convenient for industrialized production.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Preparing method and application of medicine composition of gamithromycin
InactiveCN106924280ALong-lasting blood concentrationLow costAntibacterial agentsOrganic active ingredientsRetention timeMedicine
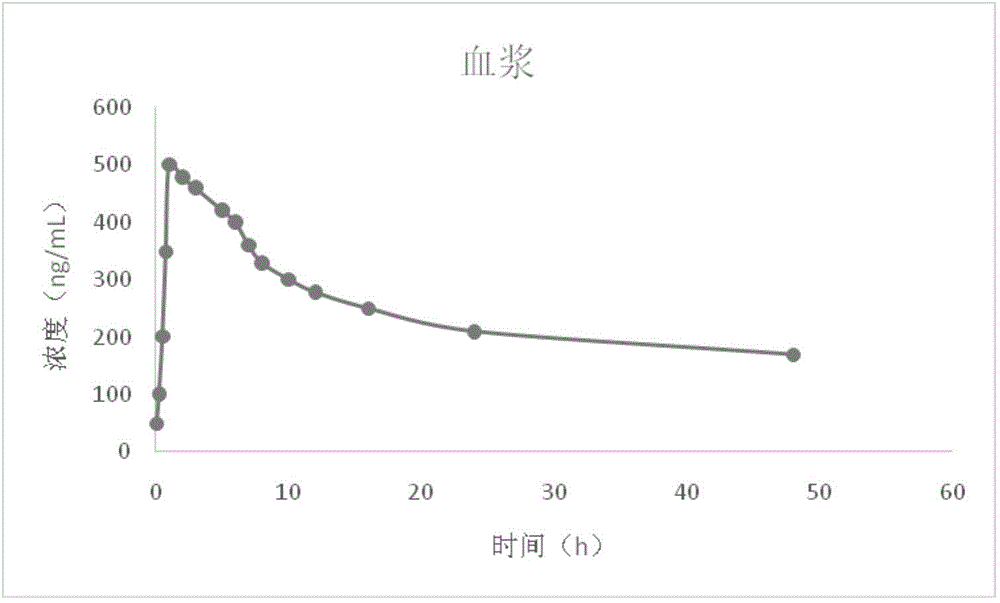
The invention provides a composition. The composition is composed of gamithromycin, polyvinylpyrrolidone and a pharmaceutically acceptable carrier. The composition has the advantages of being long in plasma concentration retention time and low in composition cost.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Gamithromycin composition lyophilized powder for injection and preparation method
ActiveCN103494780BLess irritatingImprove stabilityAntibacterial agentsPowder deliveryAdditive ingredientSuccinic acid
Owner:QILU ANIMAL HEALTH PROD
Method for detecting gamithromycin content
InactiveCN107632102AImprove stabilityGood repeatabilityComponent separationDipotassium hydrogen phosphateUv detector
The invention provides a method for detecting the gamithromycin content. The method is high performance liquid chromatography, a C18 chromatographic column is used to enforce separation, a UV detectoris used to carry out quantitative analysis, and chromatographic conditions are as follows: the mobile phase comprises a dipotassium hydrogen phosphate solution with the pH value being 7.8-8.2 and theconcentration being 0.01 mol / L and 60-80% (v / v) of acetonitrile; the flow velocity is 0.8-1.2 mL / min; the column temperature is 35-45 DEG C; and the detection wavelength is 210 nm. The composition, the kind, the concentration and the ratio of the mobile phase are selected in the method to reach a high sensitivity and a high separation degree by using the UV detector, so the problems of high priceand use convenience, brought by selection of an evaporative light-scattering detector for improving the sensitivity and the separation effect in the prior art, are solved. The method has the advantages of simplicity, easiness in operation, accurate and reliable result, high sensitivity, good reappearance, high separation degree, low cost, cheap and easily available device and reagents, and convenience in promotion and application.
Owner:华北制药集团动物保健品有限责任公司 +1
A hybridoma cell line secreting gamimycin monoclonal antibody and its application
ActiveCN111778215BHigh detection sensitivityImprove featuresDepsipeptidesTissue cultureImmune profilingImmuno detection
The invention discloses a hybridoma cell line secreting gamimycin monoclonal antibody and its application, belonging to the field of food safety immunoassay. The hybridoma cell line ABC11 secreting gamimycin monoclonal antibody of the present invention has been deposited in the General Microbiology Center CGMCC of the China Microbiological Culture Collection Management Committee, with a storage date of November 28, 2019, and a storage number of CGMCC No.19167. The gamimycin monoclonal antibody secreted by the strain is used for the analysis and detection of gamimycin residues in food safety testing. The garamimycin monoclonal antibody cell line obtained by the present invention can be used for immunoassay detection, and it has better detection sensitivity and specificity (IC 50 The value is 0.82ng / mL, less than 10% crossover to gamithromycin analogues, crossover rate=(IC of gamithromycin 50 / analogue IC 50 )×100%).
Owner:JIANGNAN UNIV +1
Preparation method of gamitomycin or its precursor 13-decladine compound
ActiveCN104693251BHigh yieldSimple processSugar derivativesSugar derivatives preparationChemical synthesisReaction temperature
The invention discloses a preparation method of gamithromycin or a 13-descladinosylation compound serving as a precursor of gamithromycin, belonging to the field of chemical synthesis. The method comprises the steps of adding a reaction substrate and a solvent into a high-pressure kettle, wherein the reaction substrate is gamithromycin or the precursor thereof, and the solvent is low-grade amide, low-grade ketone or low-grade alcohol; adding acid, and controlling the pH value of a reaction system at 0.5-5; introducing nitrogen gas to the high-pressure kettle, and keeping the pressure at 0.1-0.8Mpa; and controlling the reaction temperature at 0-35 DEG C and stirring for 1-12 hours. The preparation method is simple in process and high in selectivity, and the yield of gamithromycin or the 13-descladinosylation compound serving as the precursor of gamithromycin is high. Gamithromycin or the precursor thereof contains hydroxyls, ester groups and a plurality of ether bonds; and the reaction substrate of gamithromycin and the precursor thereof in the method disclosed by the invention selectively breaks the ether bonds at the juncture of cladinose and a matrix under an acid condition, but the hydroxyls and the ester groups are unchanged under the reaction condition, so that the selectivity is high.
Owner:QILU SYNVA PHARMA
Gamithromycin microemulsion for injection and preparation method thereof
ActiveCN113813230AImprove stabilitySimple manufacturing processAntibacterial agentsOrganic active ingredientsOil phaseMicroemulsion
The invention discloses a gamithromycin microemulsion for injection and a preparation method thereof. The preparation method comprises the following steps: adding an oil phase solvent and gamithromycin or gamithromycin salt into reaction equipment with a preset temperature, performing stirring until the mixture is dissolved, and then performing cooling to room temperature; adding an emulsifier, a co-emulsifier and water for injection into the reaction equipment until a preparation is homogeneous and stable to obtain an intermediate; and carrying out sterilization treatment on the intermediate to obtain a finished product. The gamithromycin microemulsion for injection comprises the following components in percentage by mass: 1%-30% of gamithromycin or salts thereof, 10%-50% of oil-phase solvent, 1.5%-10% of emulsifier, 1.5%-10% of co-emulsifier and 1%-85% of water for injection. The gamithromycin microemulsion for injection provided by the invention is simple in preparation process and relatively low in cost, the prepared finished product is high in stability, and the injection irritation is within a safe range.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
A kind of gamimycin injection and preparation method thereof
ActiveCN112386572BGood solubilization effectAntioxidantAntibacterial agentsOrganic active ingredientsGamithromycin Injectable SolutionVitamin C
The invention discloses a gamimycin injection and a preparation method thereof. The gamimycin injection is composed of gamimycin, vitamin C and water for injection, wherein every 100ml of the solution contains 5-35 g of gamimycin , vitamin C 1~10g, all the other are water for injection; Gamithromycin injection of the present invention uses vitamin C to improve the dissolving speed of gamimycin bulk drug, has increased the solubility of gamimycin bulk drug, and vitamin C not only It has a good solubilizing effect, and also has anti-oxidation effect, which can improve the stability of the product; the gamimycin injection of the present invention is a weakly acidic aqueous phase injection, which can be used in compatibility with most drugs, greatly increasing the Gamithromycin has stable properties after long-term storage, has little irritation to the injection site, fast absorption, remarkable effect, high safety, simple prescription, convenient quality control of raw and auxiliary materials, cost saving, and is suitable for industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Method for determining gamithromycin related substances
PendingCN114878703AAvoid product qualityComprehensive detectionComponent separationOther chemical processesPhysical chemistryGamithromycin
The invention provides a method for determining gamithromycin related substances, and belongs to the technical field of column chromatography test or analysis materials. The gamithromycin related substances refer to impurities introduced in a gamithromycin synthesis process or generated by degradation. The method is applied to determination of gamithromycin related substances, and has the advantages of stable chromatographic condition system, good linearity, high precision, high chromatographic condition sensitivity, good specificity, stable determination solution within 35 h, and the like.
Owner:ZHEJIANG GUOBANG PHARMA
A kind of synthetic method of gamithromycin
ActiveCN112300221BAvoid generatingHigh puritySugar derivativesSugar derivatives preparationOrganic acidBiochemical engineering
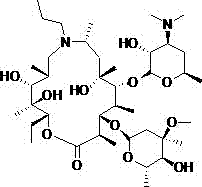
The invention discloses a method for synthesizing gamithromycin, which uses 9-deoxy-8a-aza-8a-homoerythromycin A as a raw material, and diaces with propionaldehyde in a protic solvent under the catalysis of an organic acid. Alcohol reaction forms propyral 9-deoxy-8a-aza-8a-homoerythromycin A, then reduces through boron reducing agent, removes protecting group under acidic conditions, obtains gamithromycin; preparation process of the present invention The yield of the obtained gamithromycin product can reach more than 92.0% before post-treatment, after recrystallization treatment, its purity can reach more than 99.0%, the overall yield can reach more than 90.0%, and the reaction process is mild, Able to realize industrialized mass production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Gamithromycin crystal form I and preparation method thereof
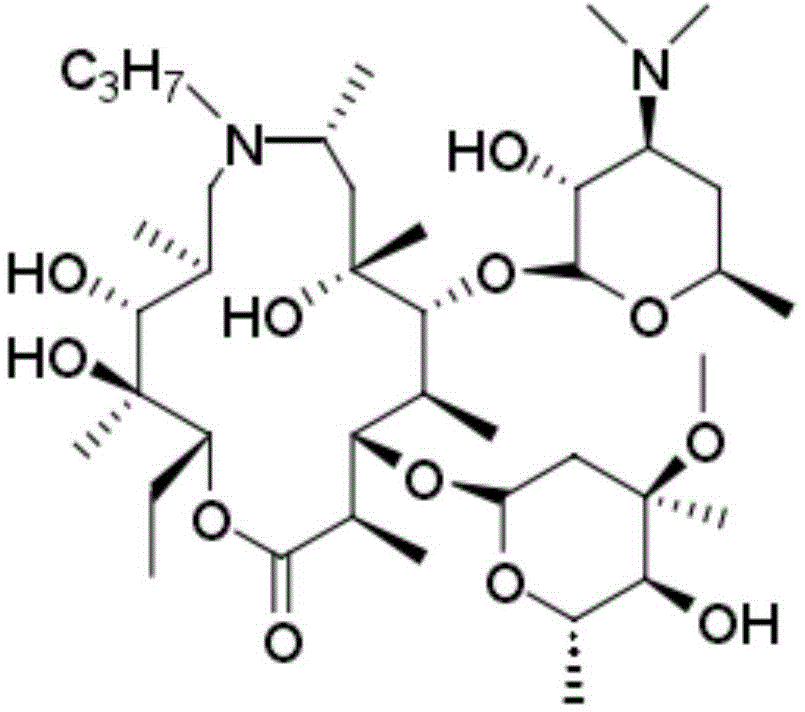
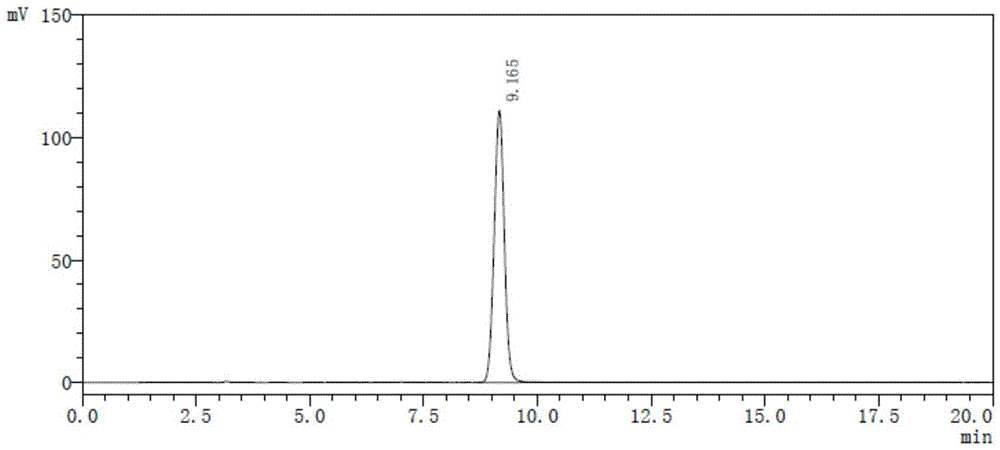
ActiveCN104788514AEasy to manufactureHigh puritySugar derivativesOrganic chemistry methodsX-rayPowder diffraction
The invention relates to a gamithromycin crystal form I and a preparation method thereof. According to the gamithromycin crystal form I, Cu-Ka radiation is used, X-ray powder diffraction shown by an angle 2(theta) is carried out, and the gamithromycin crystal form I has characteristic absorption peaks at 6.8165 degrees + / - 0.3 degree, 9.5249 degrees + / - 0.3 degree, 10.1349 degrees + / - 0.3 degree, 11.4490 degrees + / - 0.3 degree, 12.9373 degrees + / - 0.3 degree, 14.9831 degrees + / - 0.3 degree, 19.2302 degrees + / - 0.3 degree and 20.5137 + / - 0.3 degree. The gamithromycin crystal form I provided by the invention is easy to prepare, and related test data shows that the gamithromycin crystal form I is high purity, low in impurity content and good in stability.
Owner:QILU SYNVA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com