Preparation method of Gamithromycin intermediate
A technology for gamycin and intermediates, applied in the field of pharmaceutical synthesis, can solve the problems of affecting product yield and quality, high pressure, and high production costs, and achieve the effects of facilitating industrial production, reducing acid degradation products, and reducing preparation costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Preparation of (9Z)-9-deoxy-9-hydroxyiminoerythromycin A
[0041] Add 1600mL isopropanol to a 2L three-necked reaction flask, control the temperature to 20°C, stir, add 200g (0.267mol) (9E)-9-deoxy-9-hydroxyiminoerythromycin A (Xinyu Chemical Industry), Stir until dissolved. Dissolve 25g (1.086moL) of lithium hydroxide (Chengdu Kelong Chemical Reagent Factory) in 400mL of purified water and introduce it into the above-mentioned three-necked flask. Concentrate to 400ml by rotary steaming, adjust the pH to 8 with 20wt% hydrochloric acid at 0°C, pour 1000ml of purified water, precipitate a white solid, dry it after suction filtration, then wash with 300ml of dichloromethane, and dissolve the filter cake in 500ml of isopropyl Alcohol, the filtrate was obtained by suction filtration, and 500ml of dichloromethane was added to recrystallize at 0°C to obtain 140g of (9Z)-9-deoxy-9-hydroxyiminoerythromycin A as a light white solid, yield: 70%; HPLC Assayed purity: ...
Embodiment 29
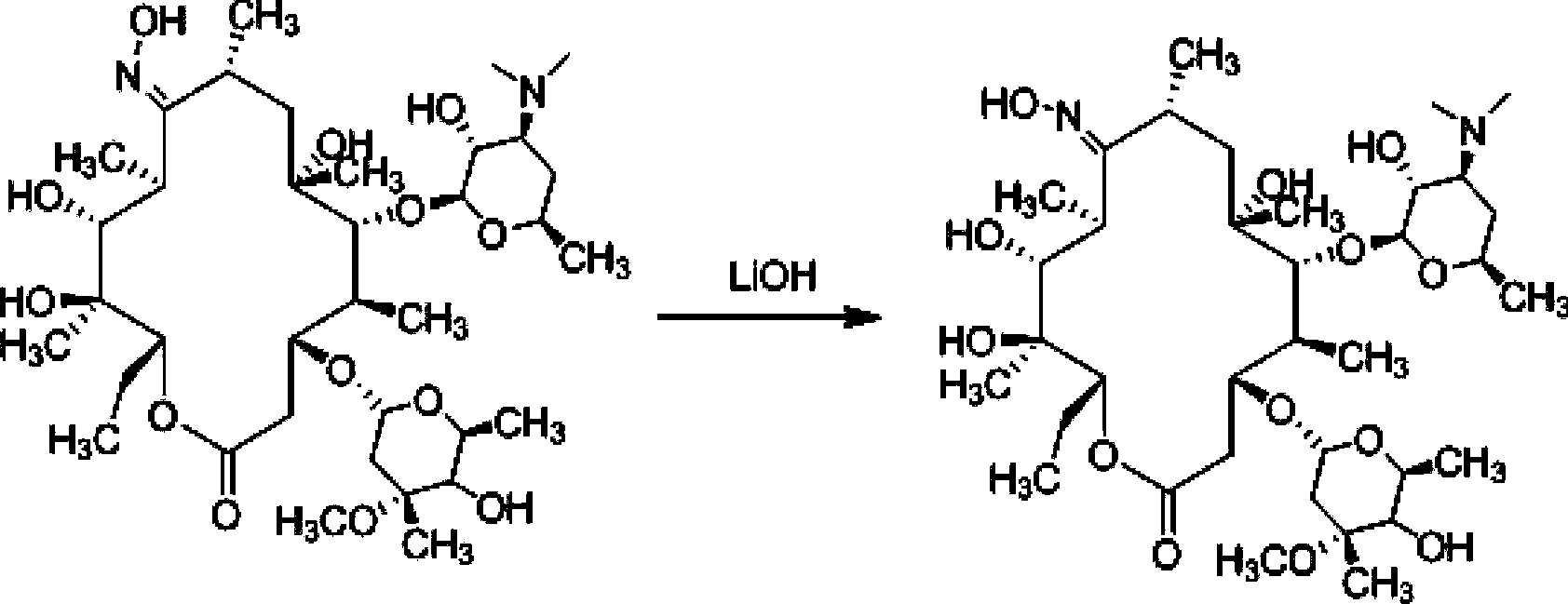
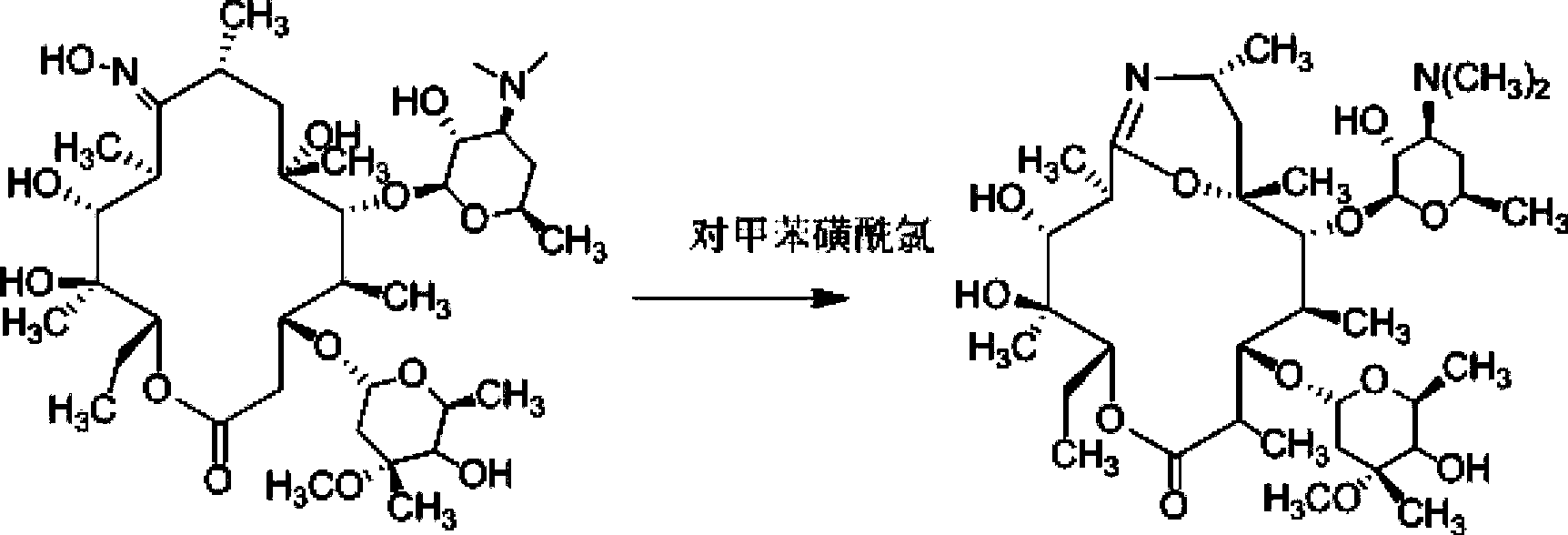
[0046] Example 2. Preparation of 9-deoxy-6-deoxy-6,9-iminoether-(8a-aza-8a)-erythromycin A
[0047] Add 1000mL acetone to a 2L three-necked reaction flask, control the temperature to -4°C, stir, add 100g (0.267mol) (9Z)-9-deoxy-9-hydroxyiminoerythromycin A, and stir until dissolved. Take 50g (1.086moL) of p-toluenesulfonyl chloride (Tianjin Damao Chemical Reagent Factory) and dissolve it in 600mL of acetone, add it dropwise at constant pressure, and finish the dropping within 20 minutes. Weigh 45g of sodium bicarbonate and dissolve it in 300mL of purified water, and add dropwise at constant pressure. The temperature was controlled at -4°C, and the reaction was carried out for 1.5 hours, and the reaction was detected by TLC. Suction filtration, the filtrate was extracted with 800ml of dichloromethane and 800ml, the organic layer was separated, dried with anhydrous sodium sulfate and evaporated to dryness to obtain a light yellow viscous liquid, which was recrystallized with ...
Embodiment 3
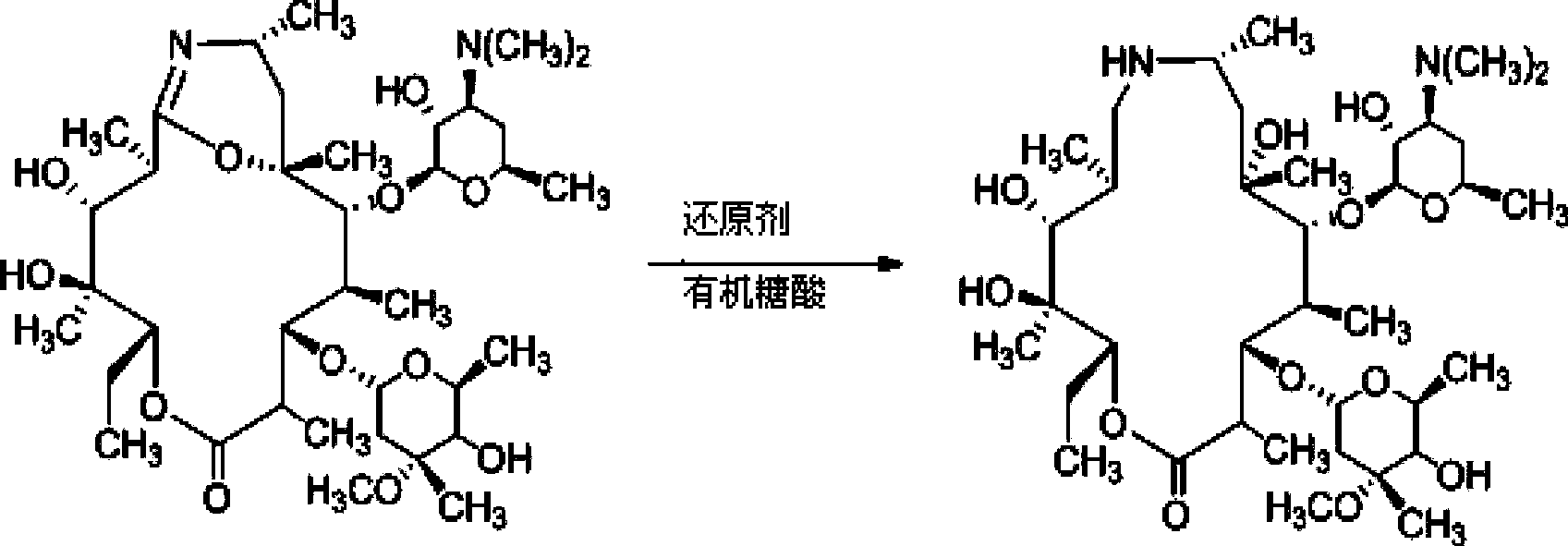
[0051] Example 3.9-Deoxy-8a-aza-8a-homoerythromycin A preparation
[0052] Add 500mL acetone to a 1L three-necked reaction flask, control the temperature to 0°C, stir, add 50g (0.267mol) 9-deoxy-6-deoxy-6,9-iminoether-(8a-aza-8a)-red Mycin A, stirred until dissolved. 20 g of sodium borohydride was added in three batches over 30 minutes. React at 0-5°C for 2.5h, rise to room temperature (25°C) for 8h. After the reaction was detected by TLC, add 300mL of water and 20wt% hydrochloric acid to adjust the pH to 3, extract with dichloromethane (120mL×3) to obtain the aqueous phase, slowly add 20wt% NaOH dropwise at 0-5°C to adjust the pH to 10, Extract with dichloromethane (180mL×3), combine the organic phases, wash the extract with saturated brine (120mL×3), dry over anhydrous magnesium sulfate, and evaporate to dryness to obtain 31g of crude product, yield: 62%. Purity determined by HPLC: 86.1%. (Application of WATERSACQUITY UPLC BEH C18 column (2.1×50mm, 1.7μm); mobile phase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com