A kind of synthetic method of gamithromycin
A technology of gamimycin and its synthesis method, which is applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc. It can solve the problems of high difficulty in purification, high toxicity of waste liquid, and medium purity of gamimycin, etc., and achieves The reaction conditions are easy to control, realize industrialized large-scale production, and improve the effect of purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
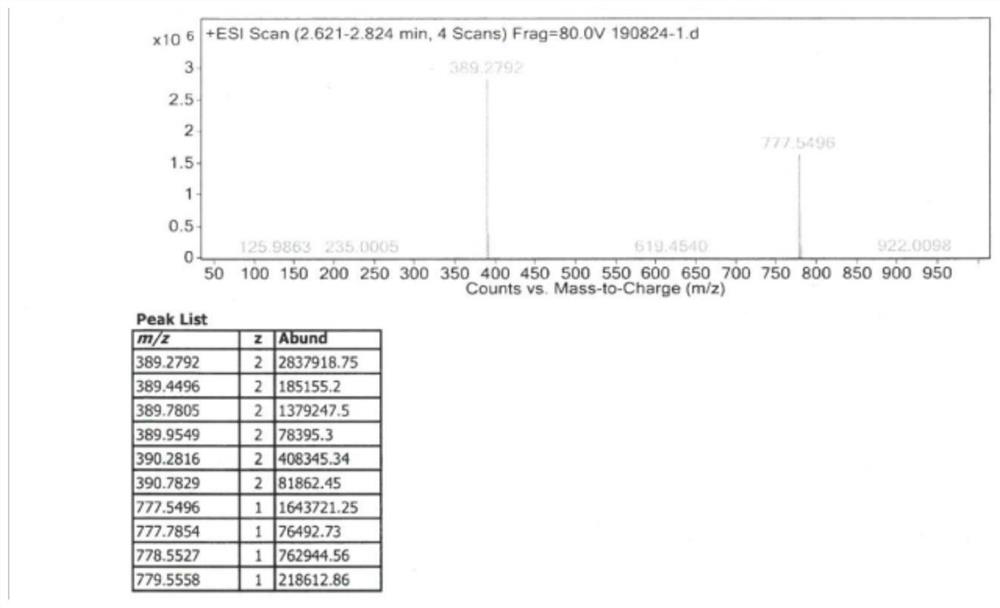
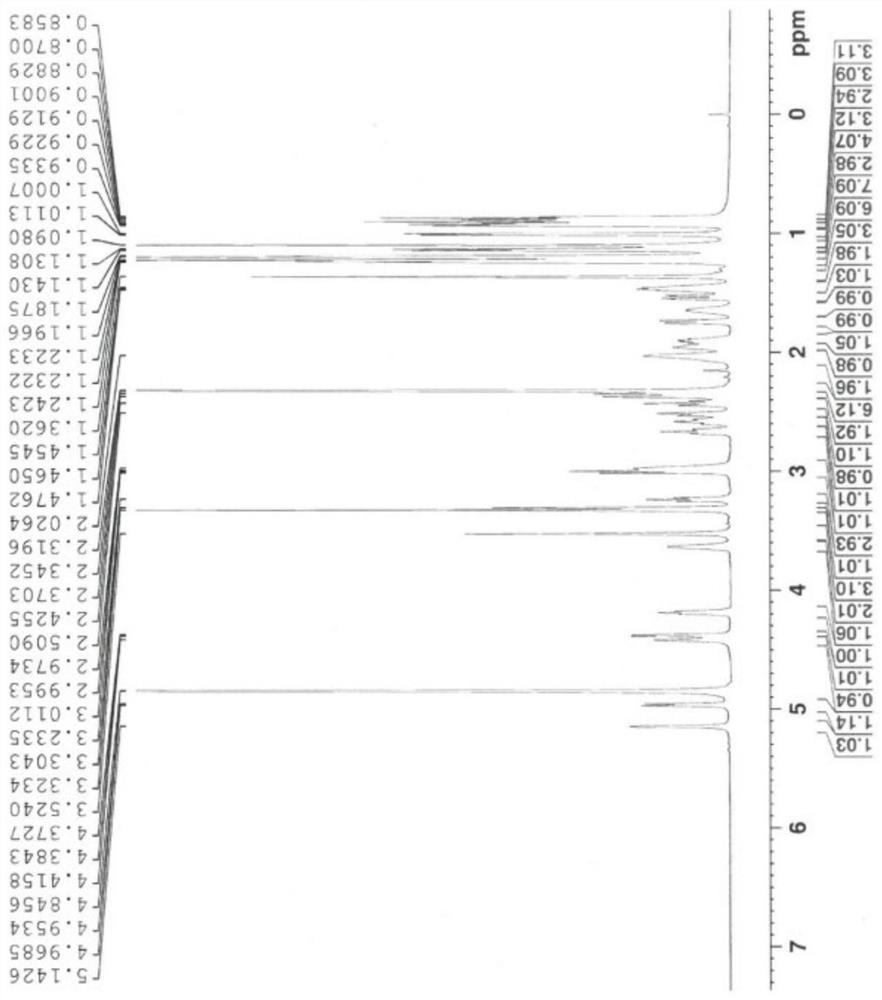
[0034] A synthetic method of gamithromycin, comprising: at room temperature, using 9-deoxy-8a-aza-8a-homoerythromycin A as a raw material, in a protic solvent, under organic acid catalysis, and propionaldehyde The diol reaction forms propyral 9-deoxy-8a-aza-8a-homoerythromycin A, which is then reduced by a boron reducing agent, and the protecting group is removed under acidic conditions to obtain gamithromycin. The invention creatively adopts propionaldehyde acetal to first protect the adjacent dihydroxyl group, and then use boron reducing agent to reduce at room temperature, avoiding the formation of gamimycin borate impurities, and improving the purity and yield of the product , due to the ingenious design of the idea of the present invention, the reaction can be carried out only at room temperature, without the need for low temperature, and the reaction conditions are easy to control, reducing energy consumption and saving costs.
[0035] Its synthetic reaction equation i...
Embodiment 1
[0056] ①Add 735g of 9-deoxy-8a-aza-8a-homoerythromycin A and 2.20L of methanol to the reaction kettle, add 23g of formic acid and 1.1kg of propionaldehyde dimethylacetal in turn under stirring, and stir at 10°C for 0.5 Hour, then add 317.8g sodium triacetylborohydride therein, react at 10 ℃ for 10 hours, obtain reaction solution;
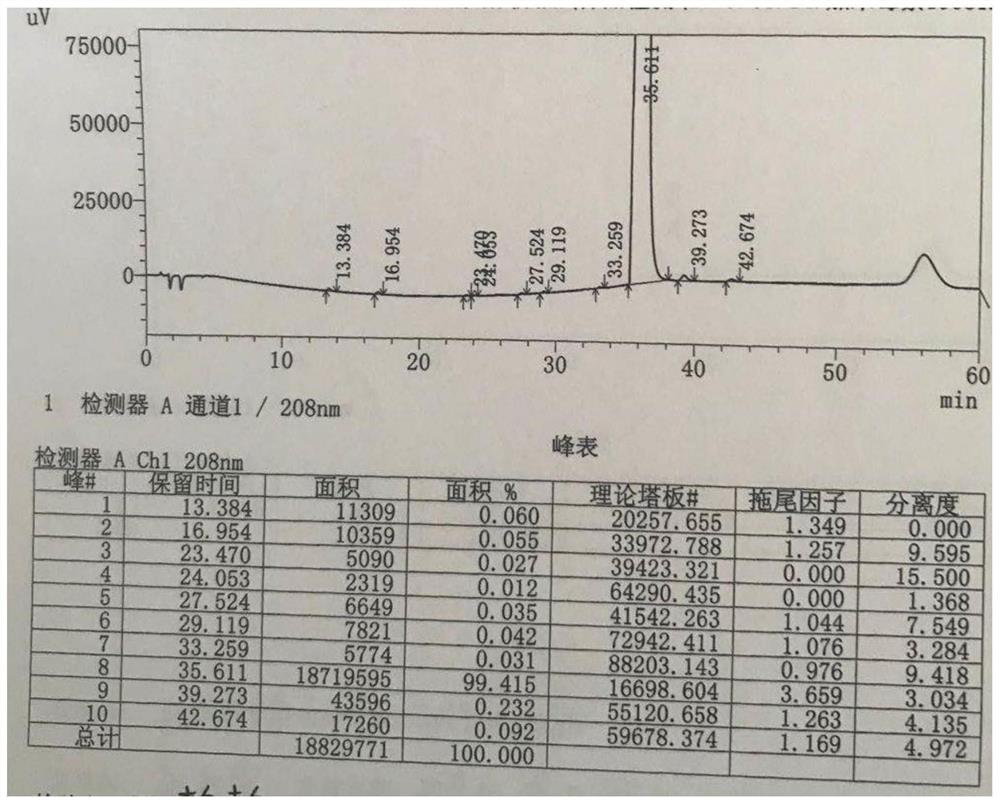
[0057] ② Add 1 times the volume of water to the reaction solution in step ① to quench the reaction, add 4.4L dichloromethane for extraction, add 4.4L distilled water to the organic phase, adjust the pH to 1 with acetic acid, stir for 20 minutes, and let stand to separate , the water layer was adjusted to pH 10 with a molar concentration of 1mol / L sodium hydroxide aqueous solution, stirred for 30 minutes, filtered, and the filter cake was vacuum-dried to obtain 718.0 g of gamithycin crude product, with a molar yield of 92.4%;
[0058] ③ Add 17.95 L of acetone aqueous solution to recrystallize the 718 g of the crude product of gaminomycin obtained in ...
Embodiment 2
[0060] ①Add 735g 9-deoxy-8a-aza-8a-homoerythromycin A and 5.88L ethylene glycol to the reaction kettle, add 148g propionic acid and 1.5kg propionaldehyde diethyl acetal successively under stirring, Stirred under the temperature for 4 hours, then added 372g of pyridine borane, and reacted at 35°C for 24 hours to obtain a reaction solution;
[0061] ② Add 2 times the volume of water to the reaction solution in step ① to quench the reaction, add 11L of ethyl acetate for extraction, add 33L of distilled water to the organic phase, adjust the pH to 4 with phosphoric acid, stir for 30 minutes, let stand for stratification, water The layer was adjusted to a pH of 11 with an aqueous sodium hydroxide solution having a molar concentration of 5 mol / L, stirred for 30 minutes, and filtered, and the filter cake was 725.0 g of gaminomycin crude product, with a molar yield of 93.3%;
[0062] 3. Add 10L acetone aqueous solution to recrystallize the gaminomycin crude product obtained in step 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com