Gamithromycin composition lyophilized powder for injection and preparation method
A technology of garamimycin and its composition, which is applied in the field of lyophilized powder and preparation of garamimycin composition for injection, which can solve the problems of high pain and irritation at the injection site of animals, high density and viscosity of injection liquid, and difficulty in administration operation Major problems, to achieve the effect of reducing the difficulty of the filtration system, easy injection, and simple and convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Gamimycin freeze-dried powder formula:
[0030]
[0031] Preparation:
[0032] Take the prescribed amount of gamimycin and citric acid, add them to the prescribed amount of water for injection, stir to dissolve, then adjust the pH value to 5.65 with sodium hydroxide, filter aseptically with a 0.22μm filter membrane, and use 10ml per bottle aseptically Subpackage.
[0033] Freeze-drying: first lower the temperature to -31~-32°C, and maintain the low temperature for 2 hours to fully freeze the sample, then turn on the vacuum system to make the vacuum of the front box reach 132Pa, and raise the temperature of the freeze-drying shelf to -1°C, Dry by sublimation until free of solvent. A sterile lyophilized powder was obtained.
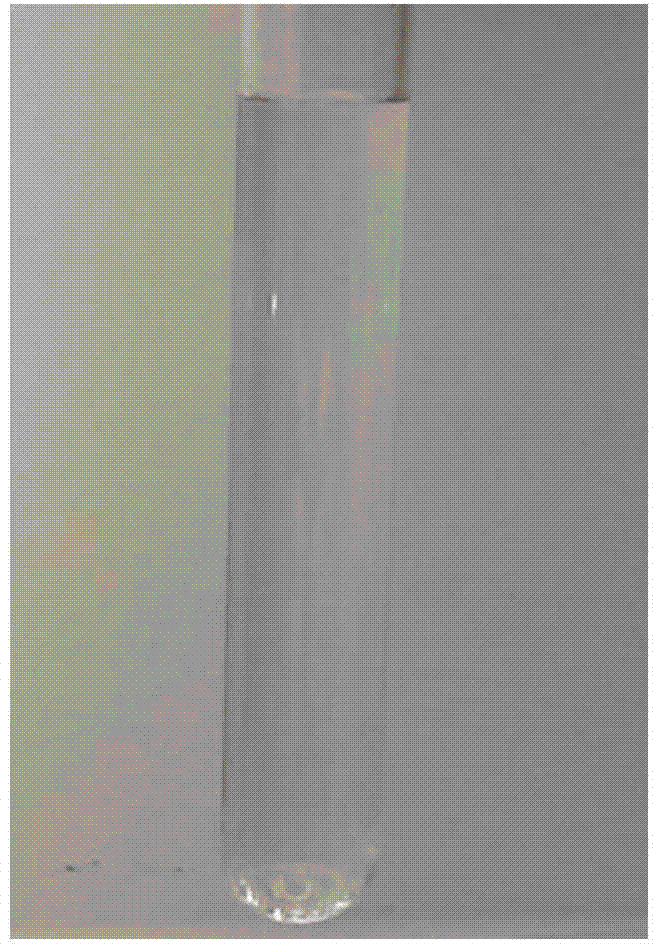
[0034] The resulting sterile freeze-dried powder is dissolved in normal saline for 24 hours and 48 hours in test tube photos as shown figure 1 , figure 2 shown.
Embodiment 2
[0036] Gamimycin freeze-dried powder formula:
[0037]
[0038] Preparation method: Take the prescribed amount of gamimycin and L-malic acid, add them to the prescribed amount of water for injection, stir to dissolve, then adjust the pH value to 5.75 with sodium hydroxide, filter aseptically with a 0.22 μm filter membrane, press 10ml sterile aliquots per bottle. The freeze-drying steps are the same as in Example 1.
Embodiment 3
[0040] Gamimycin freeze-dried powder formula:
[0041]
[0042] Preparation:
[0043] Take the prescribed amount of gamimycin and DL-tartaric acid, add them to the prescribed amount of water for injection, stir to dissolve, then adjust the pH value to 5.66 with sodium hydroxide, filter aseptically with a 0.22 μm filter membrane, and use 10ml per bottle Bacteria packing. Then, first lower the temperature to -30°C and maintain the low temperature for 3 hours to fully freeze the sample, then turn on the vacuum system to make the vacuum of the front box below 133Pa, raise the temperature of the freeze-dried laminate to -1.5°C, and carry out sublimation drying to nothing. solvent to obtain a sterile lyophilized powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com