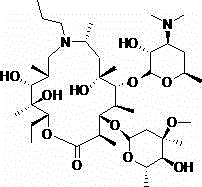
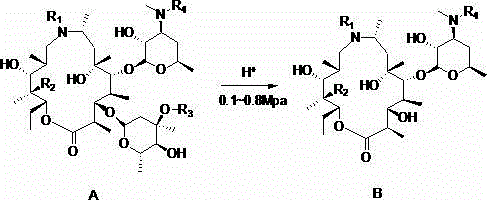
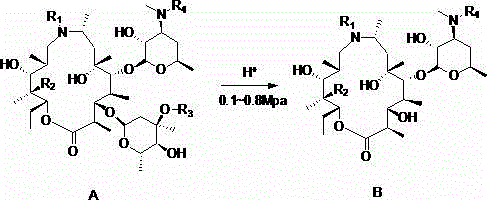
Preparation method of gamithromycin or 13-descladinosylation compound serving as precursor of gamithromycin
A technology of gamimycin and precursors, applied in the direction of preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., to achieve the effect of simple process, high selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 250ml autoclave, add gamimycin 7.8g (10mmol) and methanol 20ml, stir to dissolve, add 4mol / L aqueous hydrochloric acid dropwise, adjust the pH between 1.5-2.0, and inject N into the autoclave 2 , keep the pressure at 0.3Mpa, control the temperature at 25°C, stir for 8 hours, add 50ml of dichloromethane and 50ml of water each, adjust the pH=9.8 with 4mol / L sodium hydroxide solution, separate the liquids to obtain the organic phase concentration, add 35ml of acetone to the concentrated residue Stir and crystallize and filter. The filter cake was vacuum-dried at 50°C and white solid B was 5.4g, with a yield of 86.0% and a purity of 99.5%.
Embodiment 2
[0028] In a 250ml autoclave, add 7.4g (10mmol) of 9-deoxy-8α-aza-8α-homoerythromycin A and 20ml of methanol, stir to dissolve, add dropwise 4mol / L hydrochloric acid aqueous solution, and adjust the pH at 1.5 Between -2.0, N 2 , keep the pressure at 0.5Mpa, control the temperature at 30°C, stir for 5 hours, add 50ml of dichloromethane and 50ml of water each, adjust the pH to 9.6 with 4mol / L sodium hydroxide solution, separate the liquids to obtain the organic phase concentration, and add 35ml of ethanol to the concentrated residue Stir and crystallize and filter. The filter cake was 5.0 g of white solid B dried under vacuum at 50°C, with a yield of 83.7% and a purity of 98.4%.
Embodiment 3
[0030] In a 250ml autoclave, add gamimycin 7.8g (10mmol) and acetone 70ml, add 4mol / L sulfuric acid dropwise, adjust the pH between 2.5-3.0, and put N into the autoclave 2 , control the temperature at 30°C, keep the pressure at 0.4Mpa, add 50ml of dichloromethane and 50ml of water after stirring for 8 hours, adjust the pH=9.8 with 4mol / L sodium hydroxide solution, separate the liquids to obtain the organic phase concentration, and add 35ml of ethanol to the concentrated residue Stir and crystallize and filter. The filter cake was vacuum-dried at 50°C and white solid B was 5.1 g, with a yield of 82.0% and a purity of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com