Method for determining gamithromycin related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments: high performance liquid chromatography, Agilent 1260; electronic balance, Mettler XSR 105; pH meter, Mettler FE28.
[0037] Reagents: disodium hydrogen phosphate dodecahydrate (analytical grade), Sinopharm; ammonia water (analytical grade), Sinopharm; acetonitrile (chromatographic grade).
[0038] Chromatographic conditions: chromatographic column Waters Xterra RP18 (4.6mm×15cm, 3.5μm); flow rate 1.0ml / min; detection wavelength 210nm; injection volume 20μL; column temperature 50°C; injector condensation temperature 4°C.
[0039] Mobile phase A: 2.8mmol / L dodecahydrate disodium hydrogen phosphate solution: acetonitrile=85:15 (v / v), adjust pH=10.5 with ammonia water; mobile phase B: 80% acetonitrile aqueous solution; dissolving solution 65% acetonitrile aqueous solution .
[0040] Gradient elution was performed according to the procedure shown in Table 1.
[0041] Table 1: Gradient elution program table
[0042] time, min 0 2 18 35 40 55 56...
Embodiment 2
[0080] Chromatographic conditions: flow rate 0.9 ml / min, other chromatographic conditions are the same as in Example 1.
[0081] Take 20 μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram.
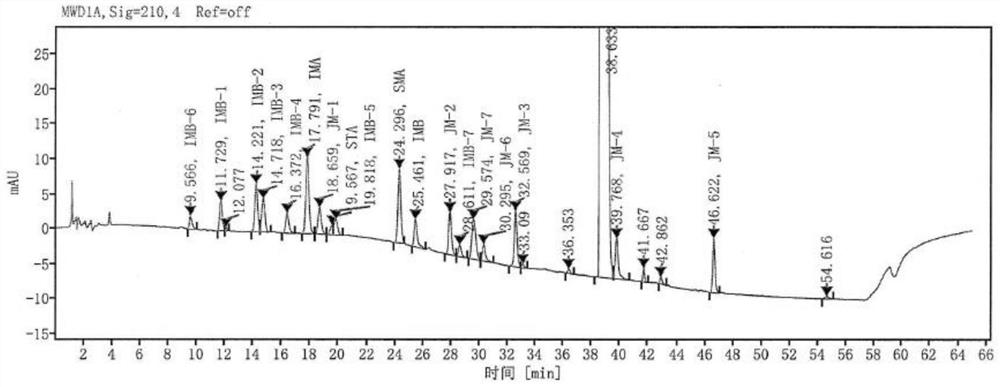
[0082] The detection results of related substances in the system suitability solution are shown in Table 10, and the obtained HPLC chromatogram is shown in Table 10. image 3 , it can be seen from Table 10 that all impurity peaks are effectively separated, and the main peak is completely separated from the adjacent impurity peaks.
[0083] Table 10: System Suitability Solution Related Substance Test Results
[0084]
[0085]
Embodiment 3
[0087] Chromatographic conditions: the flow rate was 1.1 ml / min, and other chromatographic conditions were the same as those in Example 1. .
[0088] Take 20 μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram.
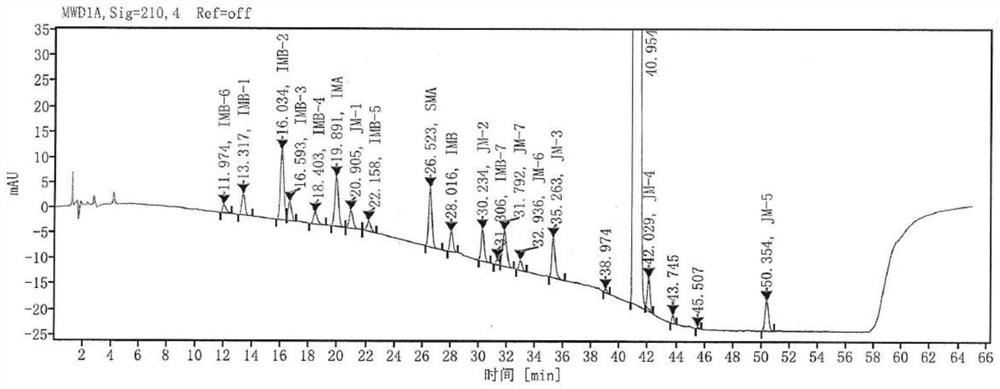
[0089] The detection results of related substances in the system suitability solution are shown in Table 11, and the obtained HPLC chromatogram is shown in Table 11. Figure 4 , it can be seen from Table 11 that all impurity peaks are effectively separated, and the main peak is completely separated from the adjacent impurity peaks.
[0090] Table 11: System Suitability Solution Related Substance Test Results
[0091]
[0092]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Filler particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com