Gamithromycin related substance and synthesis and separation method thereof
A technology of gamycin and related substances, applied in the field of gamycin related substances and synthesis and separation thereof, can solve problems such as lack of gamycin structure confirmation, and achieve the effect of improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
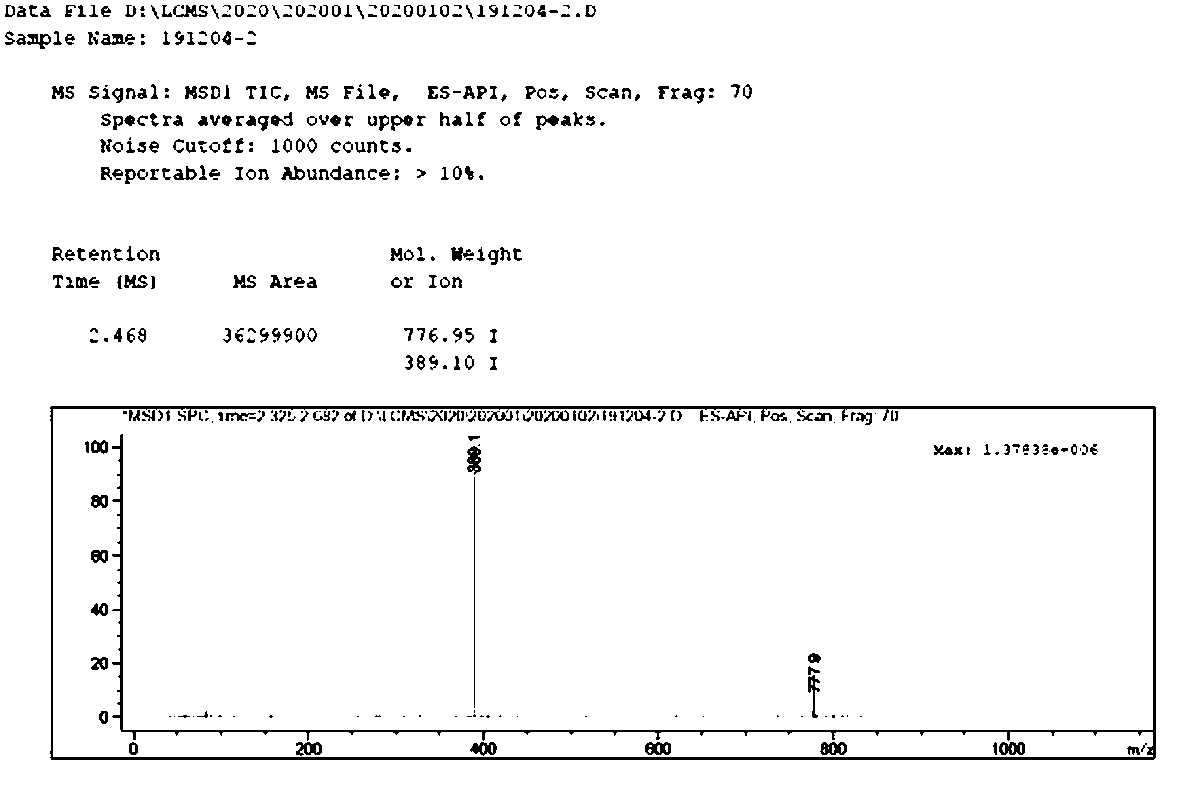
[0029] In a 250mL autoclave, add 50mL of ethanol to 5g of demethylated azithromycin, dropwise add 15mL of propionaldehyde, add 3g of 5% Pd / C, replace the air with nitrogen three times, then replace the nitrogen with hydrogen three times, control the hydrogen pressure to 0.3MPa, The hydrogen pressure range is 0.1-0.6Mpa, the temperature of the temperature control system is 35-45°C, and the reaction is 4h;
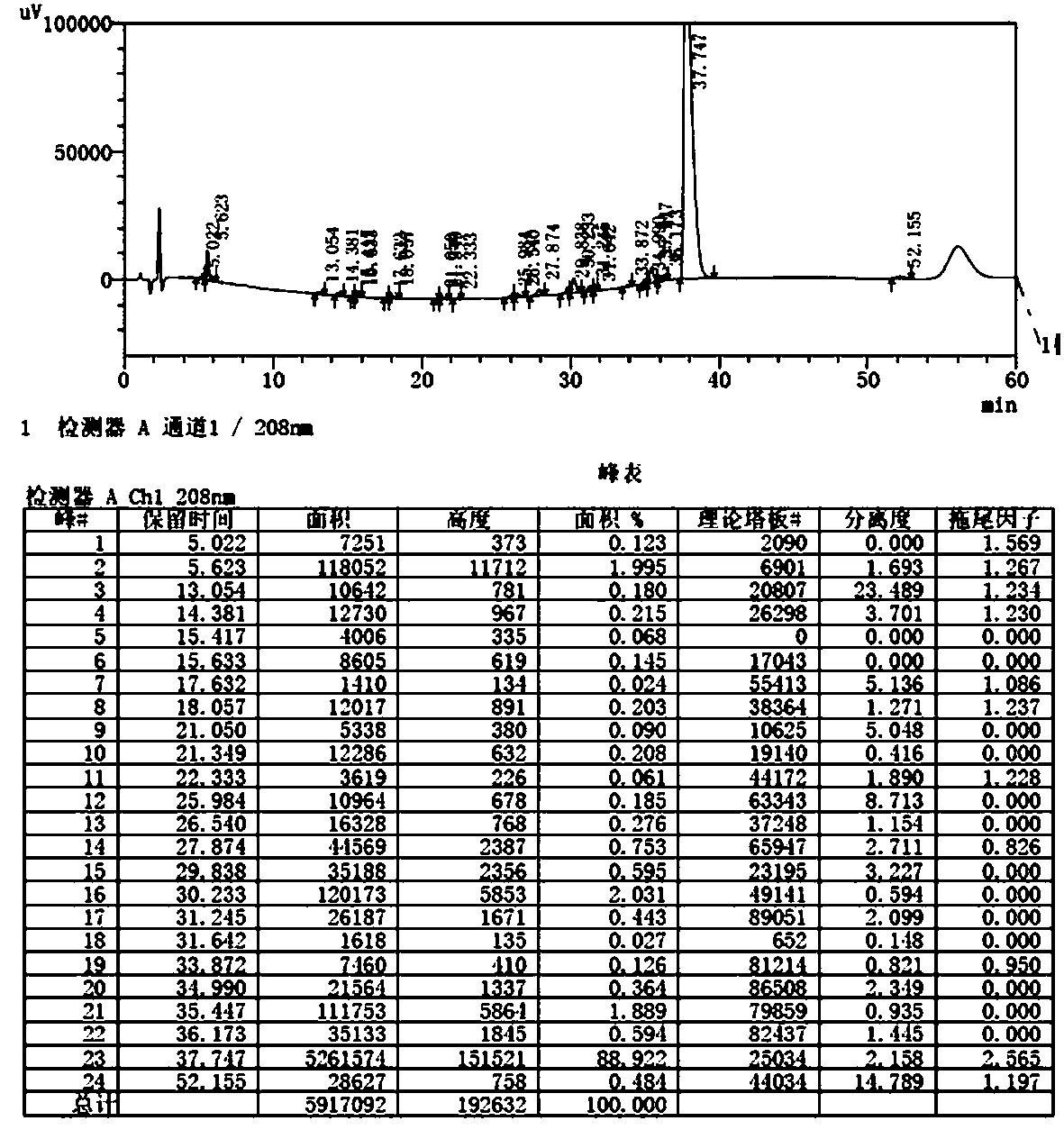
[0030] Stop the reaction, cool to room temperature, replace the gas in the reactor with nitrogen for three times, filter, wash the filter cake with ethanol 2x10mL; evaporate the ethanol to dryness under reduced pressure, add 50mL DCM and 50mL water, adjust the pH to 10.56 with 1M aqueous sodium hydroxide solution, divide layer; the organic phase was washed with 50 mL of water, and evaporated to dryness to obtain 4.84 g of an oily substance, namely N-propyl azithromycin crude product, with a purity of 84%;
[0031] Separation with a conventional silica gel column: dissolve th...
Embodiment 2
[0033] In a 250mL autoclave, add 25mL of methanol to 5g of demethylated azithromycin, dropwise add 5mL of propionaldehyde, add 2.5g of 10% Pd / C, replace the air with nitrogen three times, then replace the nitrogen with hydrogen three times, and control the hydrogen pressure to 0.1MPa , the temperature of the temperature control system is 20-35°C, and the reaction is 6h;
[0034] Stop the reaction, cool to room temperature, replace the gas in the reactor with nitrogen three times, filter, wash the filter cake with methanol 2x10mL; evaporate the methanol to dryness under reduced pressure, add 50mL DCM and 50mL water, adjust the pH to 9 with 1M aqueous sodium hydroxide solution, divide layer; the organic phase was washed with 50 mL of water, and evaporated to dryness to obtain 4.32 g of an oily substance, namely, the crude product of N-propyl azithromycin with a purity of 73%;
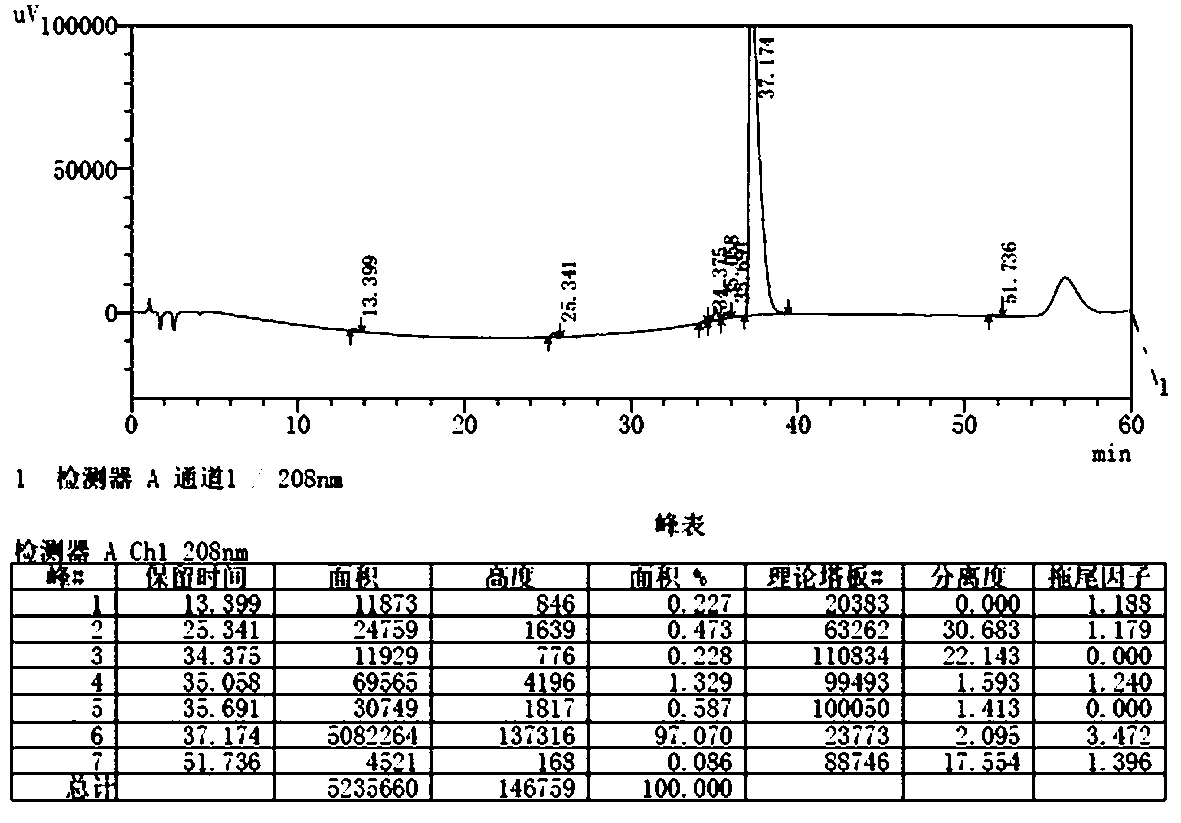
[0035] Separation with a conventional silica gel column: dissolve the crude N-propyl azithromycin obta...
Embodiment 3
[0037] In a 250mL autoclave, add 75mL of isopropanol to 5g of demethylazithromycin, dropwise add 25mL of propionaldehyde, add 5g of 5% Pd / C, replace the air with nitrogen three times, then replace the nitrogen with hydrogen three times, and control the hydrogen pressure to 0.6 MPa, the temperature of the temperature control system is 45-50°C, and the reaction is 10h;
[0038] Stop the reaction, cool to room temperature, replace the gas in the reactor with nitrogen three times, filter, wash the filter cake with isopropanol 2x10mL; evaporate the isopropanol to dryness under reduced pressure, add 50mL DCM and 50mL water, adjust the pH with 1M aqueous sodium hydroxide solution =10.95, separate layers; the organic phase was washed with 50 mL of water, and evaporated to dryness to obtain 4.93 g of an oily substance, namely N-propyl azithromycin crude product, with a purity of 84%;
[0039]Separation with a conventional silica gel column: dissolve the crude N-propyl azithromycin obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com