Gamithromycin monocrystalline type substance and preparation method thereof
A technology of gamimycin and single crystal, which is applied in the field of gamimycin single crystal and its preparation, can solve problems such as unreported crystal structure, and achieve a method suitable for large-scale industrial production with obvious bioavailability simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A kind of preparation method of gamithromycin single crystal form, concrete steps are as follows:
[0023] (1) 1 g of gamimycin white solid with a purity of 99% was placed in 15 ml of acetone, and the white solid was heated and dissolved at 40° C.;
[0024] (2) After the white solid was completely dissolved, it was cooled and placed still, crystals were precipitated, and 0.85 g of the product was obtained by filtration, with a yield of 85%.
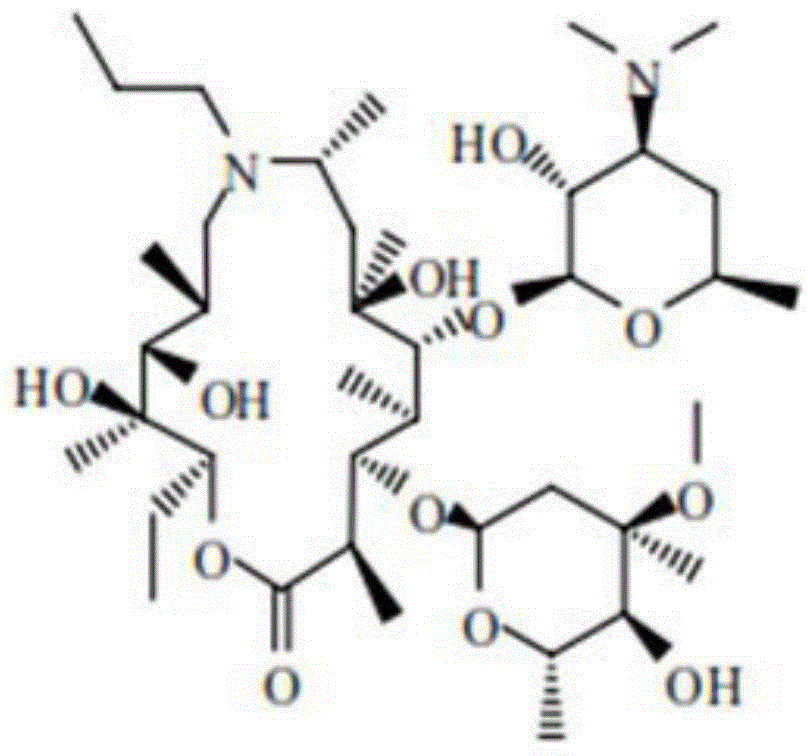
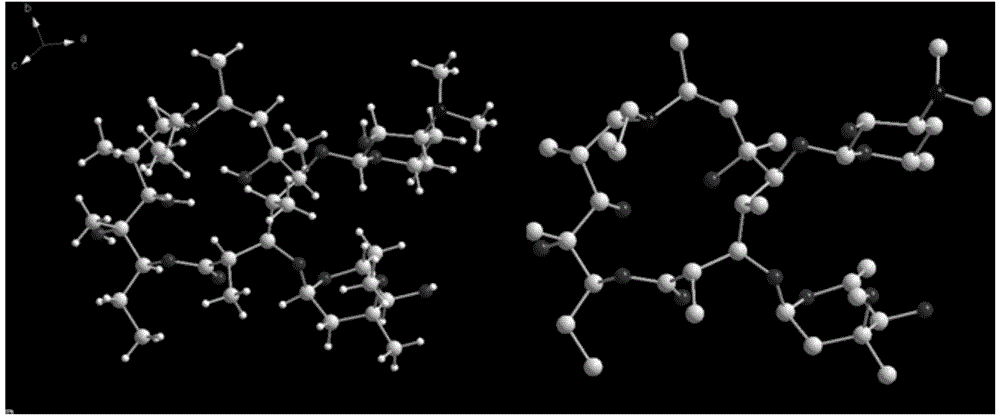
[0025] The structural formula of Gamithromycin in this embodiment is as figure 1 As shown, the obtained product has a crystal structure as measured by X-ray diffraction figure 2 As shown, it is a monoclinic crystal system, C2 space group, with chirality, and the unit cell parameters are as follows in Table 1:
[0026] Table 1
[0027]
[0028] a R 1 =Σ||F o |–|F c || / |F o |. b wxya 2 =[Σw(F o 2 –F c 2 ) 2 / Σw(F o 2 ) 2 ] 1 / 2
Embodiment 2
[0030] A kind of preparation method of gamithromycin single crystal form, concrete steps are as follows:
[0031] (1) 1g of 99% pure gamithromycin white solid is placed in 20ml organic solvent, and the white solid is heated and dissolved at 70° C., and the organic solvent is a mixture of acetonitrile and water, wherein the volume ratio of acetonitrile and water is 5: 1;
[0032] (2) After the white solid was completely dissolved, it was cooled and placed still, crystals were precipitated, and 0.7 g of the product was obtained by filtration, with a yield of 70%.
Embodiment 3
[0034] A kind of preparation method of gamithromycin single crystal form, concrete steps are as follows:
[0035] (1) 1 g of gamimycin white solid with a purity of 98% was placed in 15 ml of methanol, and the white solid was heated and dissolved at 40° C.;
[0036] (2) After the white solid was completely dissolved, it was cooled and placed still, crystals were precipitated, and 0.7 g of the product was obtained by filtration, with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com