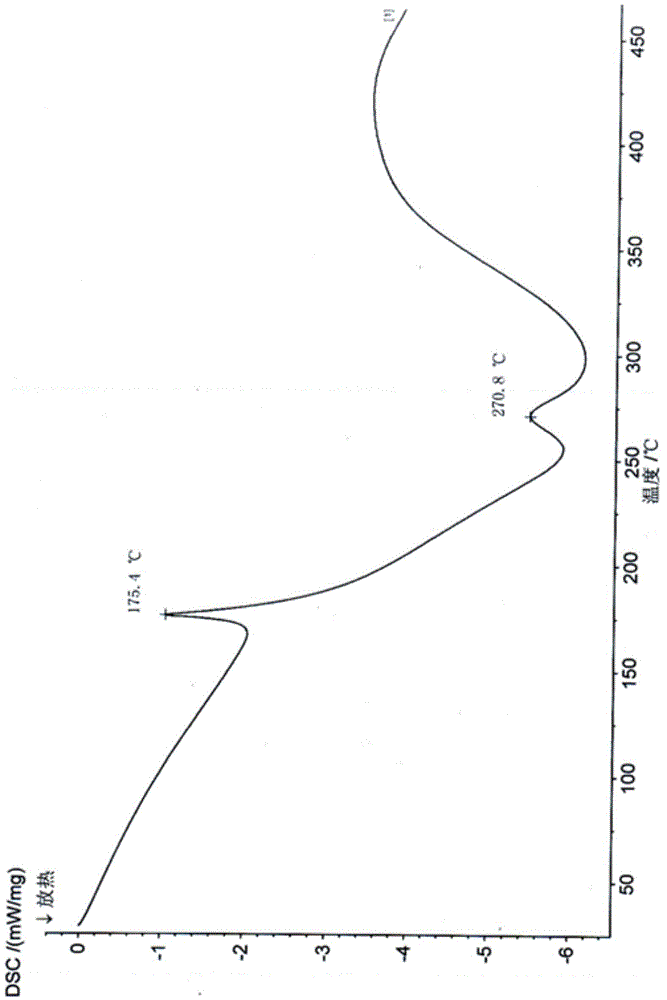
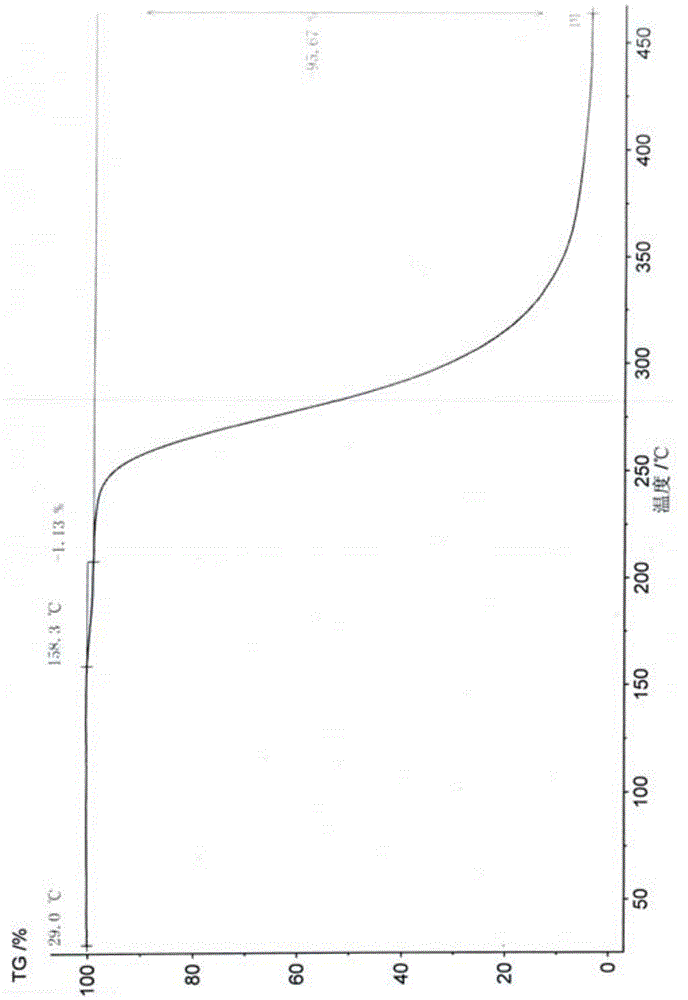
Gamithromycin crystal form I and preparation method thereof
A technology of gamimycin and its crystal form, which is applied in the field of gamimycin crystal form I and its preparation, can solve the problem of solubility and other physical and chemical properties, drug leached substances and differences in bioavailability, and can not meet the requirements of gamimycin crystal form Requirements, affecting drug stability, bioavailability and other issues, to achieve the effect of low preparation cost, easy control and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] At 60°C, add 10 g of gamimycin to a 500 ml three-neck flask, add 50 ml of isopropyl acetate, stir until the gaminomycin is completely dissolved, and then slowly add 200 ml of n-heptane dropwise. After the dropwise addition was completed, the temperature was lowered to 25°C and the crystal was grown with stirring for 2 hours, then filtered under reduced pressure, the filter cake was washed with 30ml of n-heptane, and vacuum-dried at 60°C for 24 hours to obtain a white crystalline powder, namely Gamithycin crystal Form I, the yield is 90%.
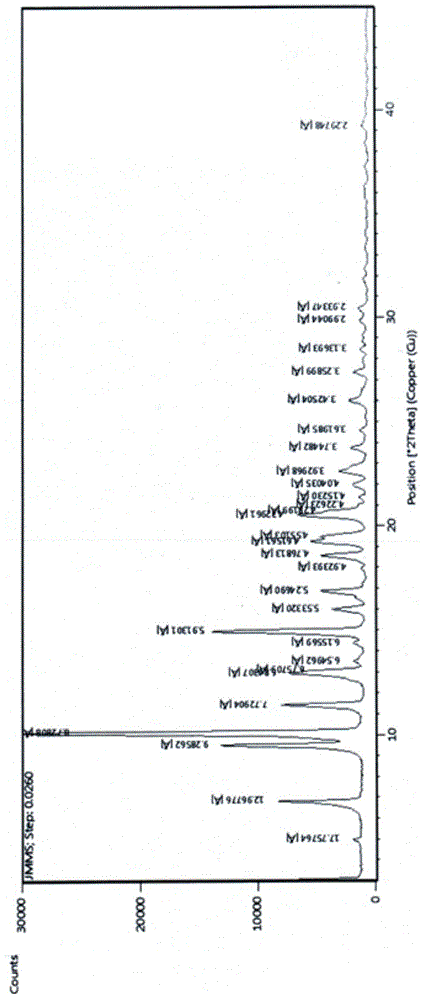
[0039] The X-ray powder diffraction pattern of gamimycin crystal form I was measured by X-ray diffractometer of PANalytical EMPYREAN in the Netherlands, the test conditions: Cu-Ka ray, 40kV, 40mA, the results are as follows figure 1 As shown, the values of the characteristic peaks in the figure are shown in Table 1:
[0040] Table 1 The characteristic peak data of the X-ray powder diffraction pattern of gamithromycin crystal form Ⅰ...
Embodiment 2
[0048] At 60°C, add 10 g of gamimycin to a 500 ml three-necked flask, add 50 ml of ethyl acetate, stir until the gaminomycin is completely dissolved, and then slowly add 200 ml of n-heptane dropwise. After the dropwise addition was completed, the temperature was lowered to 25°C and the crystal was grown with stirring for 2 hours, then filtered under reduced pressure, the filter cake was washed with 30ml of n-heptane, and vacuum-dried at 60°C for 24 hours to obtain a white crystalline powder, namely Gamithycin crystal Form I, the yield is 85%.
[0049] There is no significant difference between the analysis results of gamimycin crystal form I prepared in Example 2 and the analysis results of gaminomycin crystal form I prepared in Example 1, wherein the characteristic absorption peaks of X-ray powder diffraction are 6.9681 °, 9.6321°, 10.2441°, 11.5745°, 13.0410°, 14.9921°, 19.2893°, 20.5751°, the stable gamithromycin crystal form I can be obtained repeatedly.
Embodiment 3
[0051] Under the condition of reflux at 90°C, add 10 g of gamimycin to a 500 ml three-necked flask, add 30 ml of ethyl acetate, stir until the gaminomycin is completely dissolved, and then slowly dropwise add it into 300 ml of n-heptane. After the dropwise addition was completed, the temperature was lowered to 25°C and the crystal was grown with stirring for 2 hours, then filtered under reduced pressure, the filter cake was washed with 30ml of n-heptane, and vacuum-dried at 60°C for 24 hours to obtain a white crystalline powder, namely Gamithycin crystal Form I, the yield was 94%.
[0052] The analysis results of the gamimycin crystal form I prepared in Example 3 are not significantly different from the analysis results of the gaminomycin crystal form I prepared in Example 1, wherein the characteristic absorption peaks of X-ray powder diffraction are 6.9652°, 9.6923°, 10.2368°, 11.6158°, 13.1116°, 15.0990°, 19.2642°, 20.5393°, the stable gamithromycin crystal form I can be obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com