Separation, preparation and purification method of gamithromycin related substance
A technology of gamycin and related substances, which is applied in the field of preparation and purification, and the separation of gamitomycin-related substances, and can solve problems such as inability to identify, incapable of gamitycin quality control, and lack of gamitomycin-related substances. , to achieve the effect of simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1. The detection method of Gamithromycin related substances
[0046] Dissolve 5 mg of the synthetic crude product of gamithromycin in 1 ml of acetonitrile, and the injection volume is 20 μl. The chromatographic parameters are: the mobile phase is a mixed system of 0.05% trifluoroacetic acid aqueous solution and acetonitrile, the flow rate is 20ml / min, the column temperature is 40°C, and the preparative chromatographic column used is Phenomenex Kintex (5 μm, 150×30mm). The gradient changes are shown in Table 1:
[0047] Table 1 The change of mobile phase with time
[0048] time (min) B% (acetonitrile) 0 10 10 25 10.1 75 12 85 12.1 15
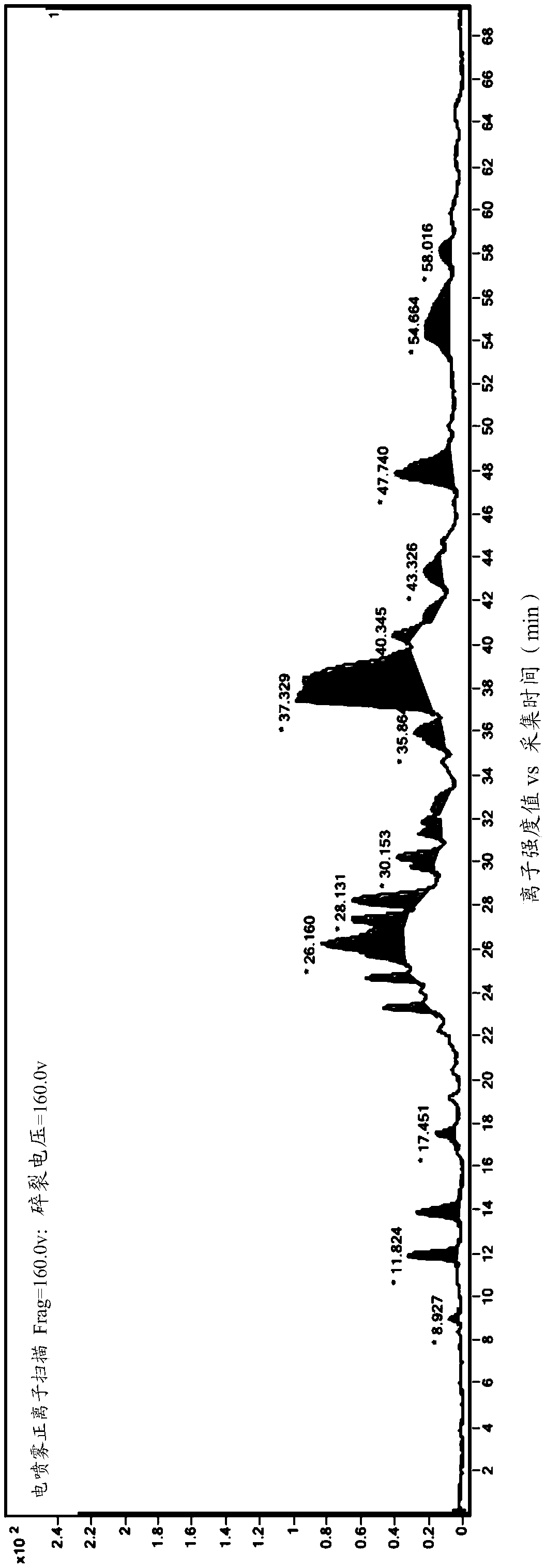
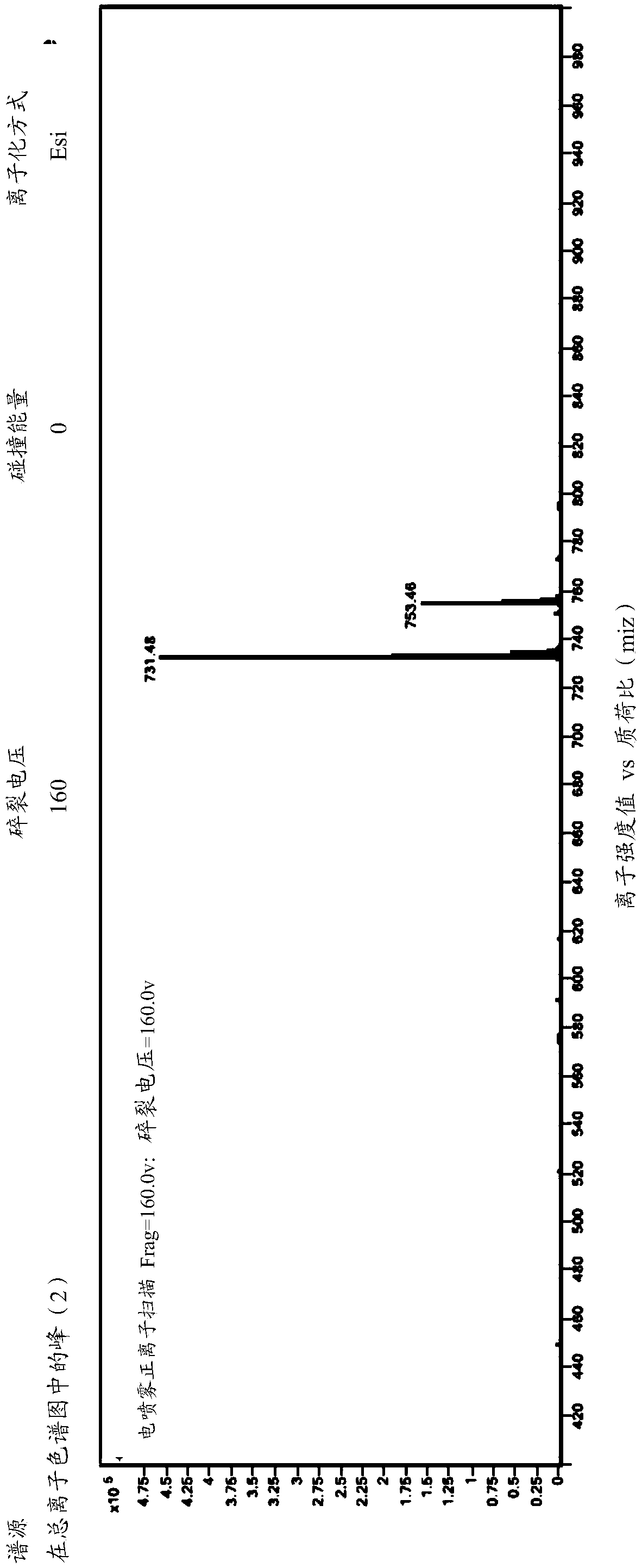
[0049] The HPLC fraction passes through the UV detector and then splits into the MS detector. The total ion flow of the mass spectrometer is as follows: figure 1 Shown, each impurity is shown in table 2 below, from figure 1 As can be seen in the above HPLC method can be used to detect...
Embodiment 2
[0054] Embodiment 2. Related substances produced by different degradation reactions of gamithromycin
[0055] 2.1 Research on acid degradation products
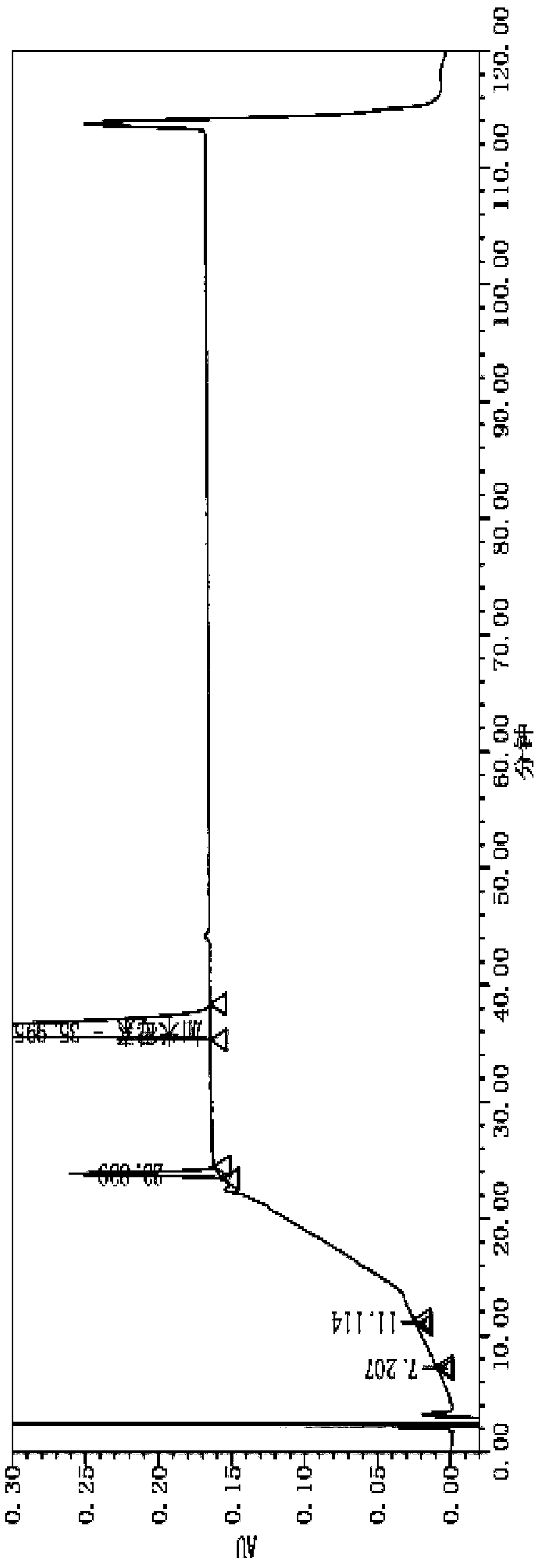
[0056] Take about 100 mg of gamimycin (batch number: 20140901), accurately weigh it, place it in a 10 mL measuring bottle, add 1 mL of 0.3 mol / L hydrochloric acid solution, let it stand at room temperature for 10 minutes, add 0.3 mol / L sodium hydroxide solution to adjust to neutral properties, add diluent to dissolve to volume. image 3 It is the HPLC spectrum of the degradation product of gamimycin acid.
[0057] 2.2 Research on Alkaline Degradation Products
[0058] Take about 100 mg of gamimycin (batch number: 20140901), weigh it accurately, put it in a 10 mL measuring bottle, add 1 mL of 1 mol / L sodium hydroxide solution, heat it in a water bath at 100 ° C for 11 h, add 1 mol / L hydrochloric acid to adjust to Neutral, add diluent to dissolve to volume. Figure 4 It is the HPLC spectrum of the degradation product of gar...
Embodiment 3
[0068] The preparation of embodiment 3 Gamithromycin related substances
[0069] Gamithromycin 10g, add 100ml of chloroform, add 2.0-5.0ml of hydrogen peroxide under stirring, heat up to 62-65°C and reflux, react for 1h, cool down to 0-5°C in an ice-water bath, add saturated sodium bisulfite solution at low temperature Quench, separate layers, and evaporate the organic phase to dryness under reduced pressure (0.1 atmosphere pressure≥vacuum degree≥0.093 atmosphere pressure, temperature 30°C) to obtain 8.8 g of crude carmimycin-related substances.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com