Method for preparing gamithromycin
A technology of gamimycin and high erythromycin, which is applied to the preparation of sugar derivatives, chemical instruments and methods, and medical preparations containing active ingredients, etc., can solve the problems of high equipment requirements and expensive reagent prices, and reduce The effect of production cost, easy scale-up production, and mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
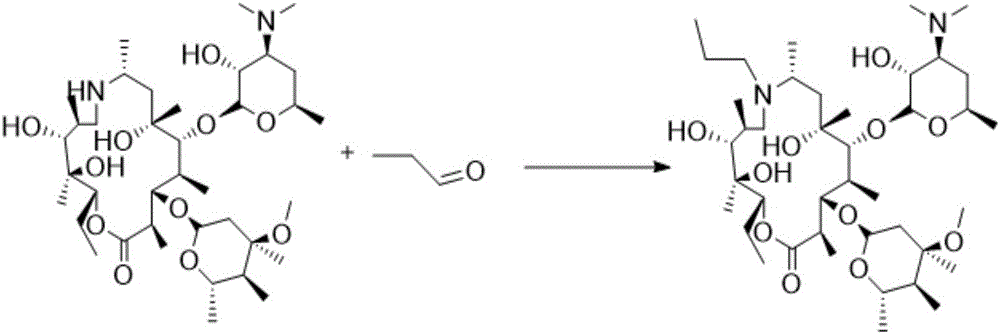
[0022] A preparation method of Gamithromycin: 9-deoxy-8a-aza-8a-homoerythromycin A (5.0g, 6.8mmol) and n-propanal (0.6g, 10.3mmol) were added in 100mL toluene, Then add 263mgPd / NiO, stir for 12 hours at 35°C under hydrogen, and detect by TLC. After the reaction is complete, filter with suction, spin dry under reduced pressure, add 60mL of water and 100mL of dichloromethane, and stir until the solids are completely dissolved. Finally, let stand to separate the liquid, discard the water layer, wash the organic layer twice, dry over anhydrous sodium sulfate, and spin dry under reduced pressure. The obtained crude product is recrystallized with methanol to obtain 4.7 g of white solid, the yield is 89.0%, and the purity by HPLC detection is 98.8 %.
Embodiment 2
[0024] A preparation method of gamithycin: 9-deoxy-8a-aza-8a-homoerythromycin A (5.0g, 6.8mmol) and n-propanal (0.6g, 10.3mmol) are added in 100mL xylene , then add 263mgPd / NiO, stir for 12 hours at a temperature of 25°C under hydrogen, and detect by TLC. After dissolving, stand for liquid separation, discard the water layer, wash the organic layer twice with water, dry over anhydrous sodium sulfate, and spin dry under reduced pressure. The obtained crude product is recrystallized with methanol to obtain 4.6 g of a white solid with a yield of 87.1%. The purity is determined by HPLC: 98.9%.
Embodiment 3
[0026] A preparation method of gamithycin: 9-deoxy-8a-aza-8a-homoerythromycin A (5.0g, 6.8mmol) and n-propanal (0.6g, 10.3mmol) were added to 100mL isopropanol Then add 263mgPd / NiO, stir for 12 hours at 35°C under hydrogen, TLC detection, after the reaction is over, filter with suction, spin dry under reduced pressure, add 60mL of water and 100mL of dichloromethane, stir until solid After complete dissolution, let stand to separate the liquid, discard the water layer, wash the organic layer twice, dry over anhydrous sodium sulfate, and spin dry under reduced pressure. The obtained crude product is recrystallized with methanol to obtain 4.8 g of white solid, the yield is 90.9%, and the purity is detected by HPLC. : 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com