Tranexamic acid sustained-release solid composition and preparation method thereof
A solid composition, tranexamic acid technology, applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., to achieve the effects of simple preparation process, maintaining blood drug concentration, and solving excessively fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
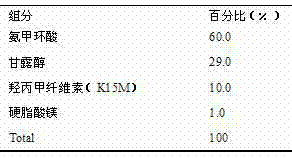
[0015] This example is presented as a tablet, wet granulated.
[0016]
[0017]
[0018] Preparation process: Weigh tranexamic acid according to the prescription amount, crush it through a 120-mesh sieve, weigh the prescription amount of mannitol and hypromellose (K15M), mix it uniformly by equal addition method, and add binder pure water Making soft material, granulating with 16 mesh sieve. Dry and measure the moisture content; granulate through a 24-mesh sieve, mix with the prescribed amount of magnesium stearate, and then mix with the granules. Ф10mm shallow concave stamping sheet.
Embodiment 2
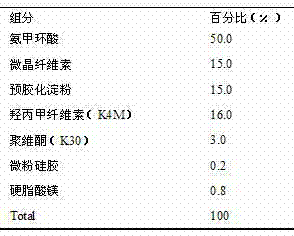
[0020] The present example is in the form of a tablet, and the powder is directly compressed.
[0021]
[0022] Preparation process: Weigh tranexamic acid according to the prescription amount, crush it through a 120-mesh sieve, weigh the prescription amount of microcrystalline cellulose, pregelatinized starch, hypromellose (K4M), povidone (K30), and use After mixing evenly by equal-volume incremental method, mix it with the prescribed amount of micropowder silica gel and magnesium stearate, and directly press the powder into tablets with a Ф12mm shallow concave punch.
[0023]
Embodiment 3
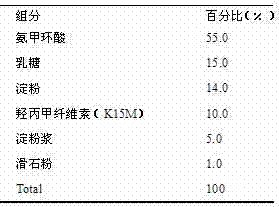
[0025] This embodiment is in the form of a capsule.
[0026]
[0027] Preparation process: Weigh tranexamic acid according to the prescription amount, crush it through a 120-mesh sieve, weigh the prescription amount of lactose, starch, and hypromellose (K15M), mix them uniformly by equal addition method, and then add 10% starch slurry Appropriate amount of soft material, granulated with 16 mesh sieve. Dry and measure the moisture content; sieve the 24-mesh sieve and mix with the prescribed amount of talcum powder to obtain the granules. 2# Capsule shell filling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com