Irbesartan hydrochlorothiazide capsule
A technology of hydrochlorothiazide and irbesartan, which is applied in the field of irbesartan hydrochlorothiazide capsules and capsules, and achieves the effect of small possibility, not easy to burst release, and safe to take
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
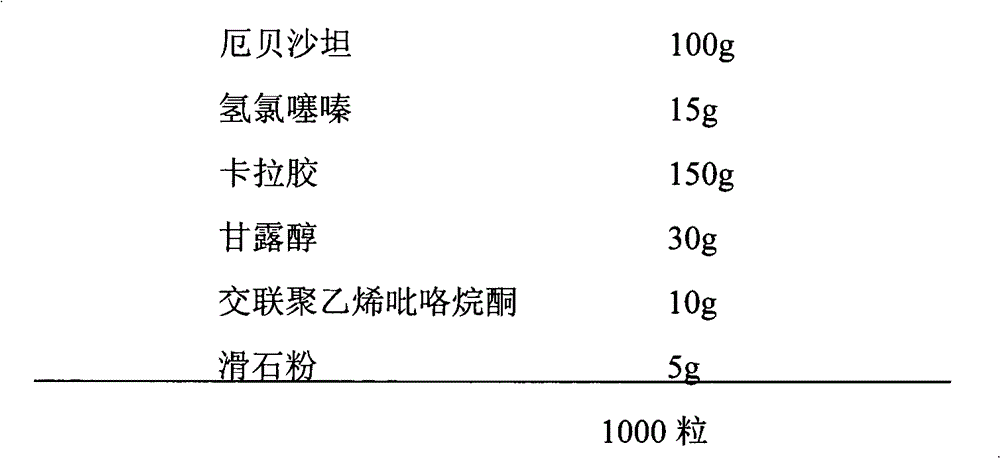
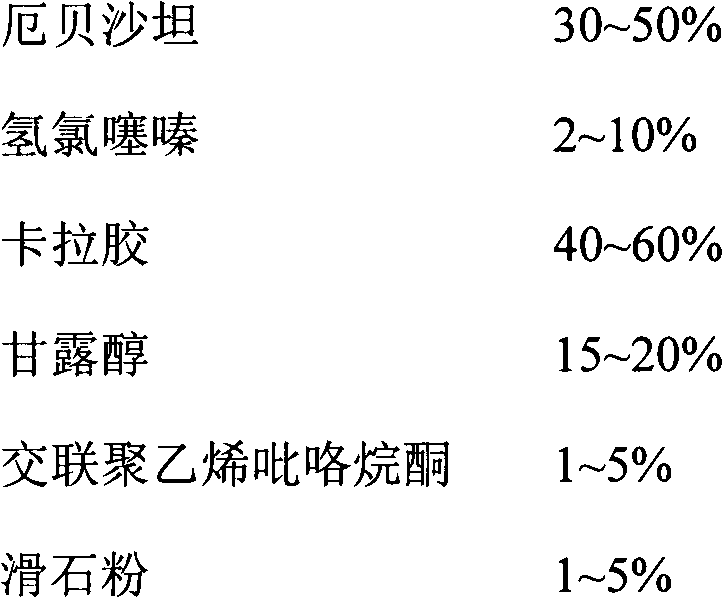
[0022]
[0023] The preparation method is as follows: Take the prescribed amount of irbesartan, hydrochlorothiazide, carrageenan, and mannitol, mix evenly, use water as the binder, granulate with 24 mesh, dry under reduced pressure at 45°C for 2 hours, granulate with 18 mesh, add the prescribed amount The cross-linked polyvinylpyrrolidone and talcum powder are mixed uniformly and filled into No. 0 capsules.
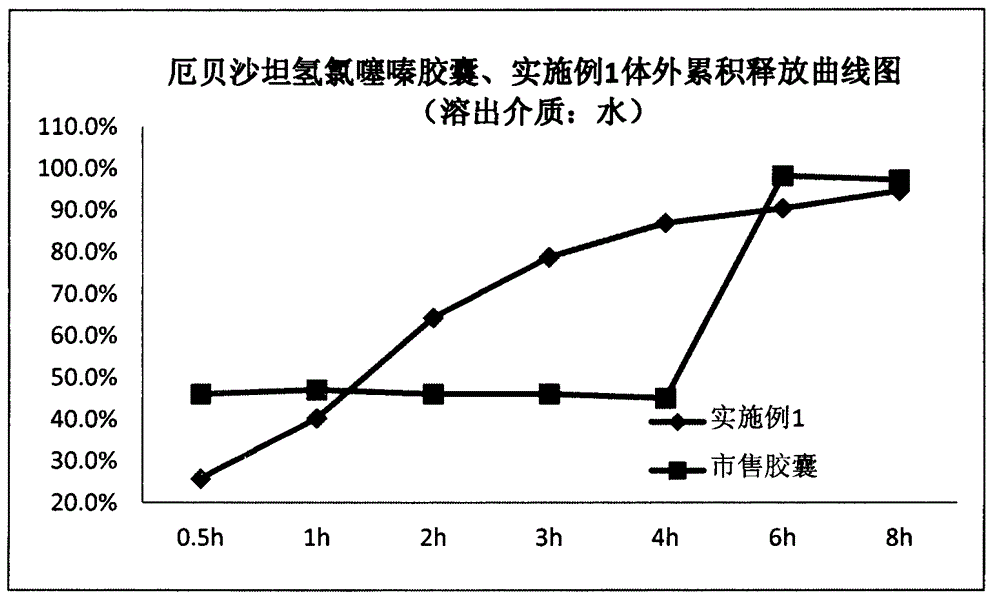
[0024] Cumulative release test results: the release of irbesartan-hydrochlorothiazide capsules prepared by the present invention is close to 0-order release, while the commercially available irbesartan-hydrochlorothiazide capsules have obvious burst release in vivo due to the presence of secondary administration.
[0025] time (h)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com