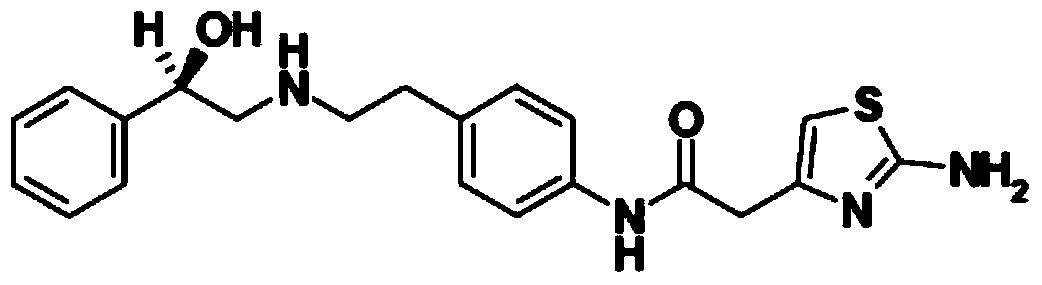
Merariveron sustained-release tablet and preparation method thereof
A technology of mirabegron and sustained-release tablets, which is applied in the field of medicine to achieve the effects of convenient taking, increased patient compliance, and increased drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
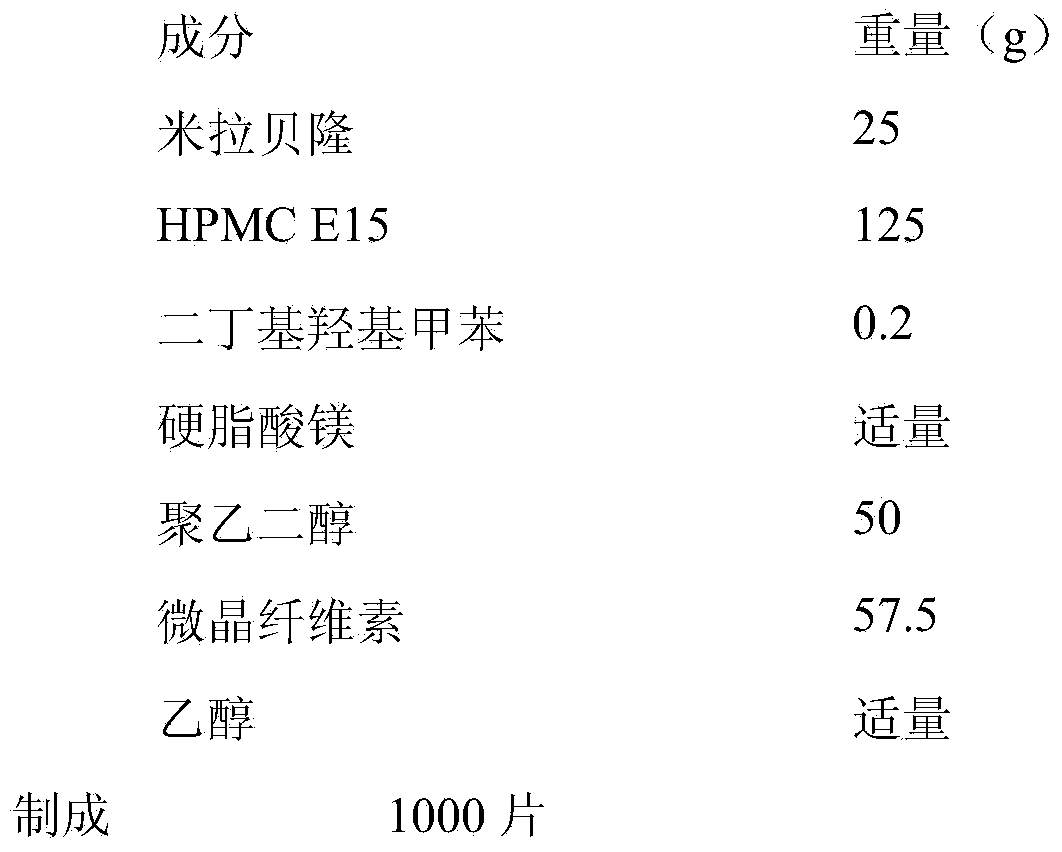
Embodiment 1
[0025]
[0026] Preparation:
[0027] (1) pass the raw and auxiliary materials through an 80-mesh sieve, and set aside;
[0028] (2) Weigh the DBT and Mirabegron of the prescription amount, mix the two evenly by the method of increasing and mixing in equal amounts, weigh HPMC E15, polyethylene glycol, microcrystalline cellulose and add and mix well 3 minutes;
[0029] (3) Add an appropriate amount of ethanol to make a soft material, pass through a 24-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0030] (4) Pass through a 26-mesh sieve for granulation, add an appropriate amount of lubricant, mix evenly, and compress into tablets.
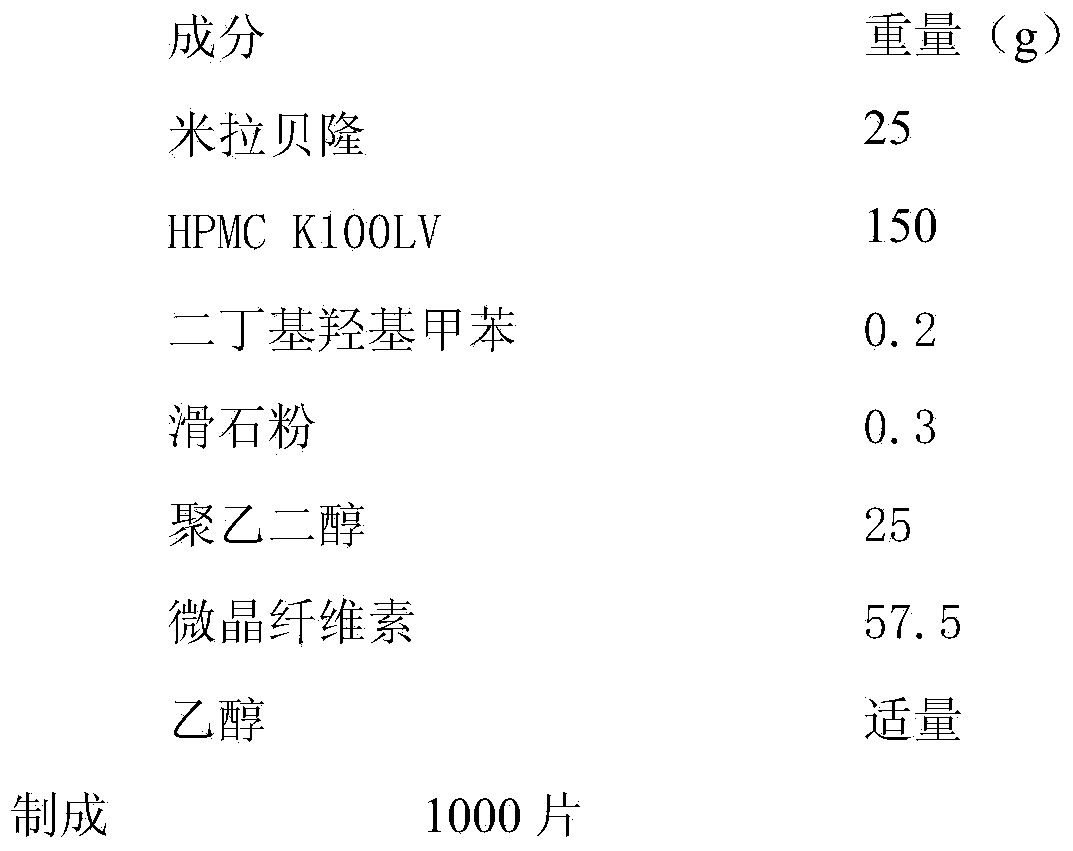
Embodiment 2
[0032]
[0033] Preparation:
[0034] (1) pass the raw and auxiliary materials through an 80-mesh sieve, and set aside;
[0035] (2) Take by weighing the DBT and Mirabegron of the prescription amount, mix the two by the method of adding and mixing in equal amounts, weigh HPMC K100LV, add polyethylene glycol and microcrystalline cellulose and mix well 3 minutes;
[0036] (3) Add an appropriate amount of ethanol to make a soft material, pass through a 24-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0037] (4) Pass through a 26-mesh sieve for granulation, add an appropriate amount of lubricant, mix evenly, and compress into tablets.
Embodiment 3
[0039]
[0040] Preparation:
[0041] (1) pass the raw and auxiliary materials through an 80-mesh sieve, and set aside;
[0042] (2) Take by weighing DTBH and Mirabegron in the prescribed amount, mix the two evenly by adding and mixing in equal amounts, weigh HPMC K4M, add polyethylene glycol and microcrystalline cellulose and mix evenly 3 minutes;
[0043] (3) Add an appropriate amount of ethanol to make a soft material, pass through a 24-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0044] (4) Pass through a 26-mesh sieve for granulation, add an appropriate amount of lubricant, mix evenly, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com