Policosanol-containing solid dispersion, controlled release tablet and preparation method thereof
A solid dispersion and policosanol technology, which is applied in the field of policosanol preparations, can solve the problems of low patient compliance, achieve the effects of prolonging the release time, improving dispersion and solubility, and reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Some embodiments of the present invention provide a method for preparing a controlled-release tablet containing policosanol as described above, comprising the following steps:
[0046] mixing policosanol, surfactant, carrier material, binder and solvent to obtain a drug-containing solution; the drug-containing solution, filler and lubricant are wet granulated to obtain drug-containing layer particles;
[0047] Provide booster layer particles and coating material solutions respectively;
[0048] Compressing the drug-containing layer granules and the booster layer granules to obtain a double-layer tablet core;
[0049] The double-layer tablet core is coated with the coating material solution, and then punched to obtain a controlled-release tablet containing policosanol.
[0050] Wherein, the specific types and dosages of the active ingredients of the drug and each auxiliary material are as described in the previous prescription. In a specific embodiment of the present i...
Embodiment 1
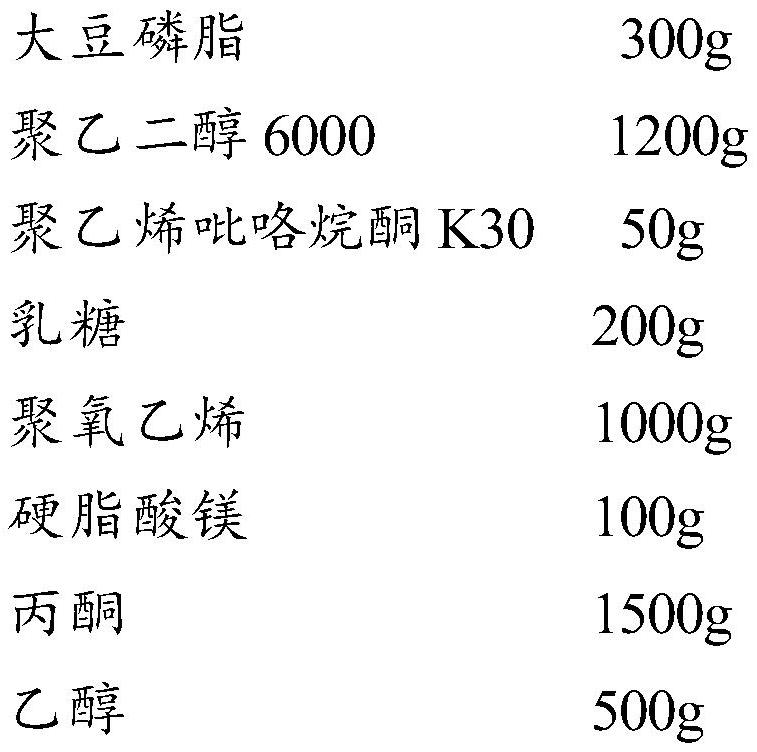
[0069] Tablet core drug-containing layer prescription:
[0070]
[0071]
[0072] Preparation process of drug-containing layer granules:
[0073] (1) Weigh the prescribed amount of soybean lecithin and polyethylene glycol 6000, respectively add them into the prescribed amount of acetone under stirring, disperse evenly and slowly raise the temperature to 50°C.
[0074] (2) Weigh the prescribed amount of policosanol, add it into the solution in (1) under stirring, and keep stirring until the policosanol dissolves to obtain a drug-containing solution.
[0075] (3) Continue heating the drug-containing solution in (2) to obtain a light yellow solid after the acetone volatilizes. After the solid is pulverized at low temperature, pass through a 100-mesh sieve.
[0076] (4) Weighing the prescribed amount of polyvinylpyrrolidone, adding it to the prescribed amount of ethanol, stirring and dissolving to obtain a binder solution.
[0077] (5) Weigh the lactose and polyoxyethylene...
Embodiment 2
[0094] Tablet core drug-containing layer prescription:
[0095]
[0096]
[0097] Preparation process of drug-containing layer granules:
[0098] (1) Weigh the prescribed amount of sucrose ester, polyethylene glycol and polyvinylpyrrolidone, respectively add them to the prescribed amount of ethanol under stirring, disperse evenly and slowly heat up to 70°C.
[0099] (2) Weigh the prescribed amount of policosanol, add it into the solution in (1) under stirring, and keep stirring until the policosanol dissolves to obtain a drug-containing solution.
[0100] (3) Take the sodium chloride and polyoxyethylene of prescription quantity, place in the fluidized bed granulator and mix. Add the solution in (2) into a fluidized bed granulator for granulation.
[0101] (4) Dry the granulated granules to obtain dry granules, add the prescribed amount of lubricant for mixing, and obtain drug-containing layer granules for future use.
[0102] Tablet Booster Layer Prescription:
[010...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com