Clonidine hydrochloride sustained-release tablet
A technology of clonidine hydrochloride and sustained-release tablets, applied in the field of medicine, can solve the problems of prolonging the biological half-life, low bioavailability, slowing down the absorption rate, etc., and achieves the effects of low cost, fewer taking times, and stable blood drug concentration
Active Publication Date: 2016-03-16
CP PHARMA QINGDAO CO LTD
View PDF4 Cites 10 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0011] In order to solve the disadvantages of inconvenient taking and low bioavailability of existing clonidine hydrochloride preparations, the present invention invents clonidine hydrochloride sustained-release tablets, which can reduce the number of times of taking the medicine, slow down the absorption rate, prolong the biological half-life, and control the blood drug concentration at the effective blood level. Within the range of drug concentration, thereby reducing side effects and improving patient compliance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1-6
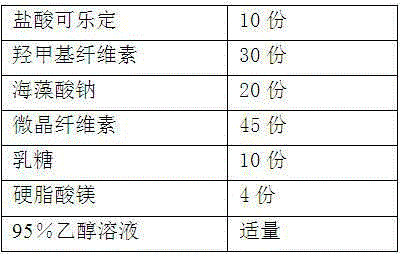
[0023] The preparation of embodiment 1-6 clonidine hydrochloride slow-release tablet
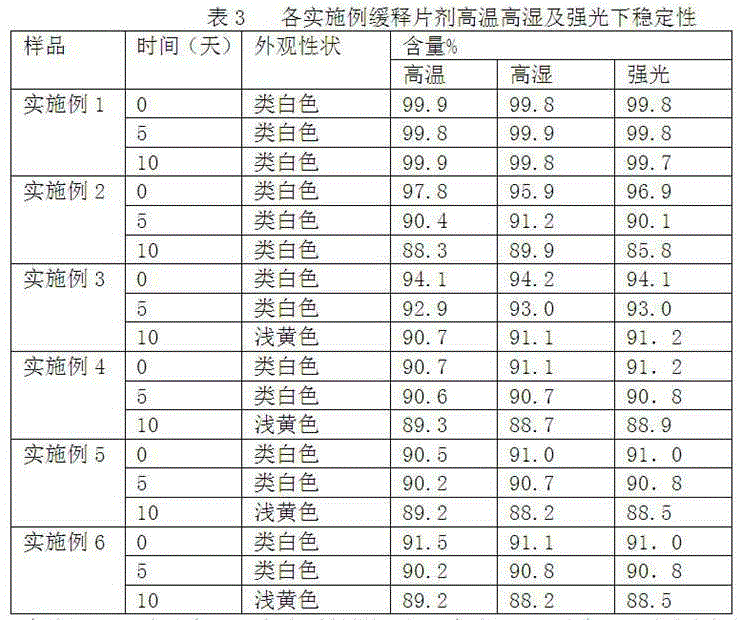
[0024] According to the raw and auxiliary materials in the following table, according to the above-mentioned preparation method, the clonidine hydrochloride sustained-release tablets of six embodiments were obtained.
[0025]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
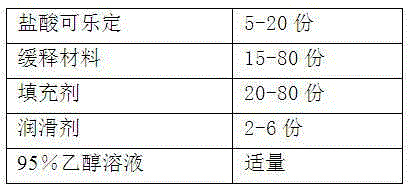
The invention provides a preparation method of a clonidine hydrochloride sustained-release tablet, and belongs to the technical field of a medicine. The clonidine hydrochloride sustained-release preparation disclosed by the invention consists of clonidine hydrochloride, a sustained-release material, a filling material, a lubricating agent and an ethanol solution with concentration being 95%. The clonidine hydrochloride, as a major ingredient, has an effect of lowering blood pressure and is effective on migraine, menopausal hectic fever and dysmenorrheal; the clonidine hydrochloride is applicable to the rapid drug treatment for addiction of opiates; and in 2010, the clonidine hydrochloride is approved to be used for treating attention deficit hyperactivity disorder in teenagers. The sustained-release preparation provided by the method is safe and effective, stable in quality, low in cost and low in administration efficiency, and the sustained-release preparation is capable of enhancing patient compliance and is capable of achieving the sustained release of drugs.
Description
technical field [0001] The invention belongs to the technical field of medicine, and relates to a method for preparing clonidine hydrochloride sustained-release tablets. The invention provides a clonidine hydrochloride sustained-release tablet that is safe, effective, stable in quality, low in cost, less in frequency of administration, enhanced in patient compliance, and capable of slowly releasing drugs. Sustained release formulations. Background technique [0002] Clonidine hydrochloride, the chemical name is 2-[(2,6-dichlorophenyl) imino] imidazolidine hydrochloride, the structural formula is: [0003] [0004] Molecular formula: C 9 h 9 C l2 N 3 ·HCl [0005] Molecular weight: 266.56 [0006] Clonidine hydrochloride directly excites the central post-synaptic membrane α2 receptors in the hypothalamus and medulla oblongata, excites the inhibitory neurons, reduces the central sympathetic nerve impulse transmission, and thus inhibits the peripheral sympathetic nerve...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/22A61K47/38A61K47/36A61K31/4168A61P29/00A61P21/02A61P9/12A61P25/36A61P25/06
CPCA61K9/0002A61K9/205A61K9/2054A61K31/4168
Inventor 王明刚陈阳生任莉孙桂玉刘晓霞杜昌余王清亭
Owner CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com