Trimetazidine dihydrochloride sustained release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release tablets, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. problems, to achieve the effect of simple steps, improved compliance, and process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 The prescription composition of trimetazidine hydrochloride sustained-release tablets is:
[0053] Raw materials
g / 100 pieces
3.5
Kollidon SR
7.0
1.75
Microcrystalline Cellulose PH101
5.1625
0.0875
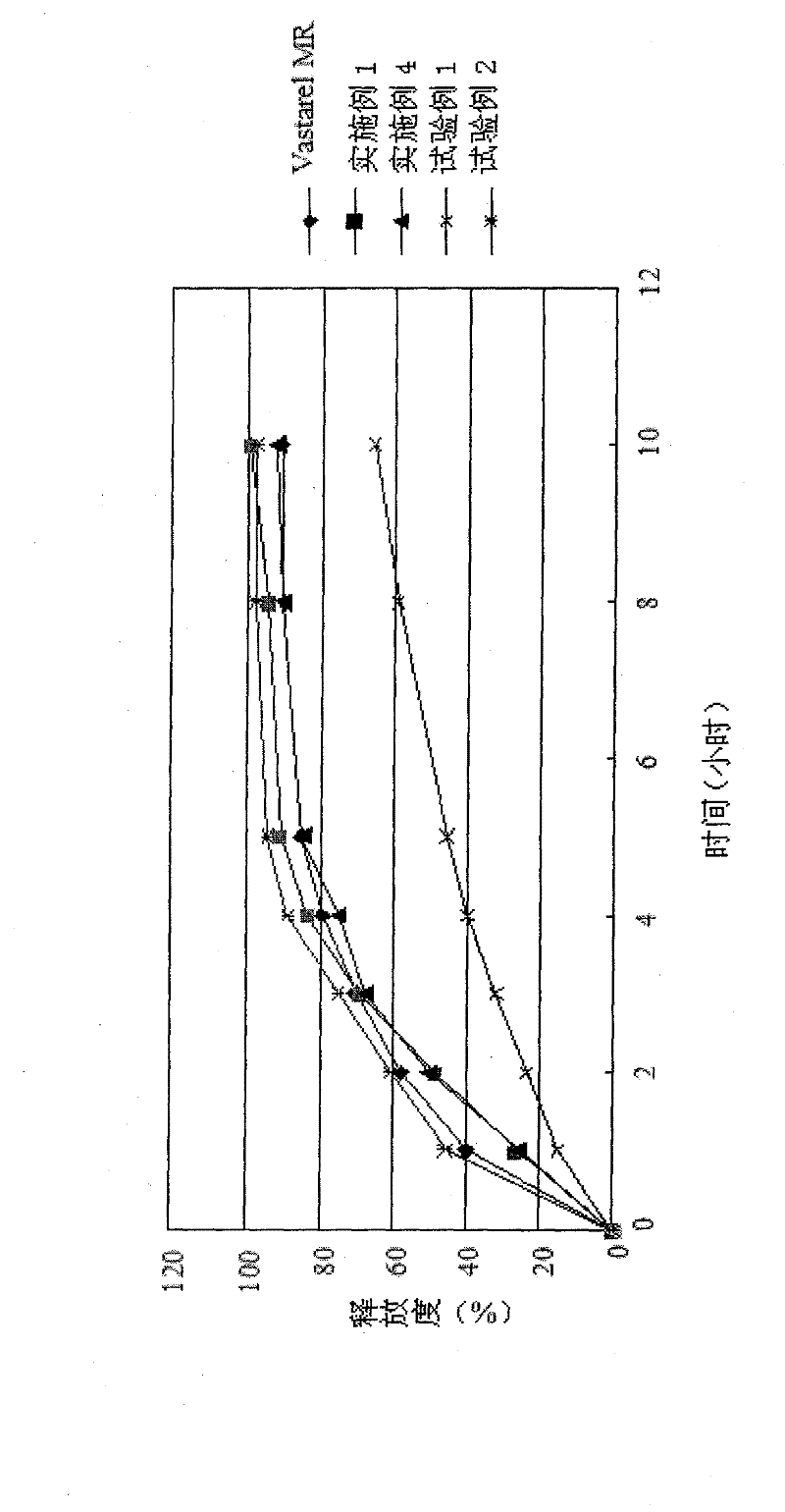
[0054] Preparation method: mix the prescription amount of trimetazidine hydrochloride with microcrystalline cellulose PH101, then mix it with Kollidon SR and ethyl cellulose uniformly, add magnesium stearate and mix and press tablets, the hardness measured is 9-10kg / mm 2 , The release degree is shown in Table 5 and figure 1 Shown.
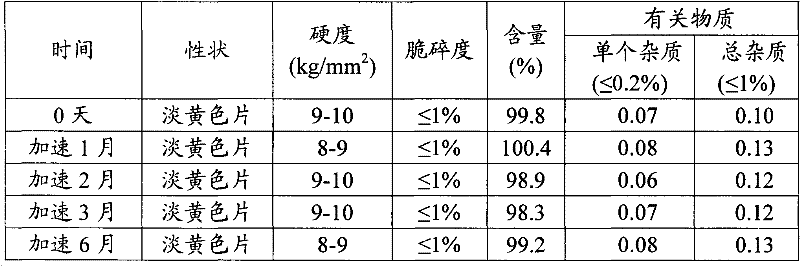
[0055] The stability of the trimetazidine hydrochloride sustained-release tablets obtained in Example 1 was studied. It was investigated under the conditions of (40±2)°C and relative humidity (75±5)%, 0 days, 1, 2, 3 And the stability of 6 months, the experiment proves that the sustained-release tablet prepared by using the mixture of Kollidon SR an...
Embodiment 2
[0058] Example 2 The prescription composition of trimetazidine hydrochloride sustained-release tablets is:
[0059] Raw materials
[0060] The preparation method: the prescription amount of trimetazidine hydrochloride is mixed with lactose, starch and ethyl cellulose, granulated with an aqueous solution of povidone, dried, and granulated. The prepared granules are mixed with Kollidon SR evenly, magnesium stearate is added and mixed, and then compressed, the hardness measured is 9-10kg / mm 2 , The measured release rate is shown in Table 2.
[0061] Table 2 Investigation of release
[0062] Time (hour)
[0063] By investigating the release profile of the trimetazidine hydrochloride sustained-release tablet obtained in Example 2, it was found that although the water-soluble filler would accelerate the release of the drug, the sustained-release time still meets the requirements.
Embodiment 3
[0064] Example 3 The prescription composition of trimetazidine hydrochloride sustained-release tablets is:
[0065] Raw materials
[0066] The preparation method: mix the prescription amount of trimetazidine hydrochloride with microcrystalline cellulose and ethyl cellulose uniformly, then granulate with a hydroxypropyl cellulose aqueous solution, dry, and sizing. The prepared granules are mixed with Kollidon SR evenly, magnesium stearate is added and mixed, and then compressed, the hardness measured is 9-10kg / mm 2 .
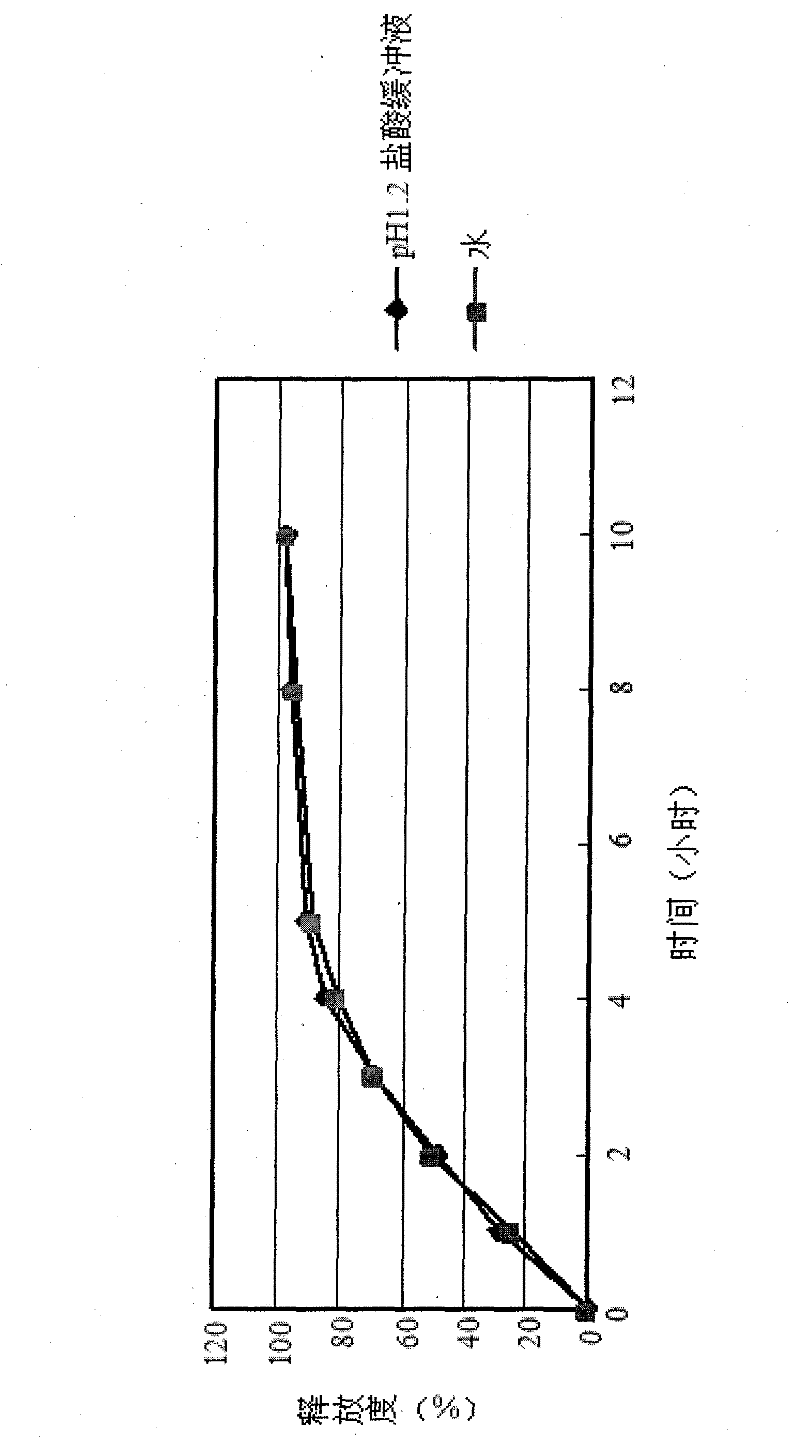
[0067] The sample obtained in Example 3 was placed in two different media with a pH of 1.2 hydrochloric acid buffer solution and water, and the release rate was measured as shown in Table 3 and figure 2 Shown.
[0068] Table 3 Investigation of release rate in different media
[0069] Time (hour)
[0070] By investigating the release curve of the trimetazidine hydrochloride sustained-release tablets obtained in Example 3, it is found that the pH environment has a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com