Memantine hydrochloride slow-release dry suspension and preparation method thereof
A technology of memantine hydrochloride and dry suspension, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, and devices that make medicines into special physical or ingestible forms, and can solve the problem of unsatisfactory slow-release dry suspension. To solve problems such as drug reporting, to achieve the effects of stabilizing blood drug concentration, solving effectiveness, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
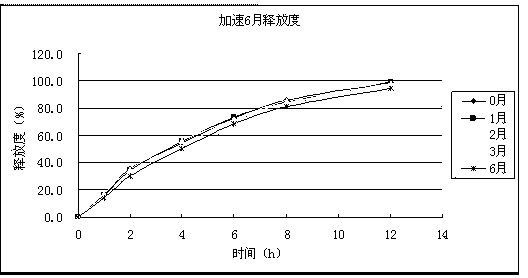
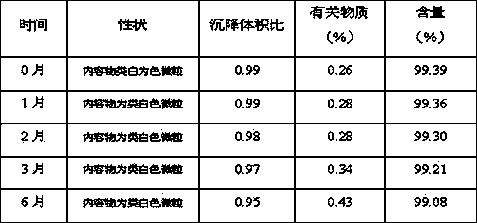
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of memantine hydrochloride sustained-release dry suspension, using the following prescription:
[0023] Raw and auxiliary materials weight (g)
[0024] Memantine Hydrochloride 28
[0025] Styrene weakly acidic cation exchange resin 90
[0026] Polyacrylic resin RS100 18
[0027] Diethyl phthalate 1.5
[0028] Polyethylene glycol 400 1.5
[0029] Lactose 136
[0030] Sucrose 195
[0031] PVP K30 10
[0032] Xanthan Gum 20
[0033] Propylparaben appropriate
[0034] Purified water
[0035] 500 in total.
[0036] Preparation Process:
[0037] (1) Preparation of memantine hydrochloride-ion exchange resin compound: add the prescription amount of styrene weakly acidic cation exchange resin to a mixer filled with deionized water, add memantine hydrochloride under agitated state and stir evenly, and continue Stir for 3 hours at a rotation speed of 600 rpm, then stand still to precipitate and filter the drug resin, remove the precipitate and place it in a tray, and place it ...
Embodiment 2
[0041] Example 2: Preparation of memantine hydrochloride sustained-release dry suspension, using the following prescription:
[0042] Raw and auxiliary materials weight (g)
[0043] Memantine Hydrochloride 28
[0044] Styrene strong acid cation exchange resin 90
[0045] Polyacrylic resin RS100 12
[0046] Dibutyl sebacate 1.5
[0047] Polyethylene glycol 400 1.5
[0048] Mannitol 136
[0049] Sucrose 201
[0050] PVP K30 10
[0051] Xanthan Gum 20
[0052] Propylparaben appropriate
[0053] Purified water
[0054] 500 in total
[0055] The preparation process and the examples are basically the same.
Embodiment 3
[0056] Example 3: Preparation of memantine hydrochloride sustained-release dry suspension, using the following prescription:
[0057] Raw and auxiliary materials weight (g)
[0058] Memantine Hydrochloride 28
[0059] Styrene strong acid cation exchange resin 90
[0060] Polyacrylic resin RS100 18
[0061] Dibutyl sebacate 1.5
[0062] Polyethylene glycol 400 1.5
[0063] Mannitol 136
[0064] Sucrose 195
[0065] PVP K30 10
[0066] Xanthan Gum 20
[0067] Propylparaben appropriate
[0068] Purified water
[0069] 500 in total
[0070] The preparation process and the examples are basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com