Dabrafenib mesylate sustained-release tablet and preparation method thereof
A technology of dabrafenib mesylate sustained-release tablets and methanesulfonic acid, which is applied in the fields of pharmaceutical formulation, coating, pill delivery, etc., can solve the problems of endangering the health of patients, forgetting to take the medicine frequently, and cumbersome taking, etc. Achieve good stability, reduce peak and valley phenomena, and reduce the number of times of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
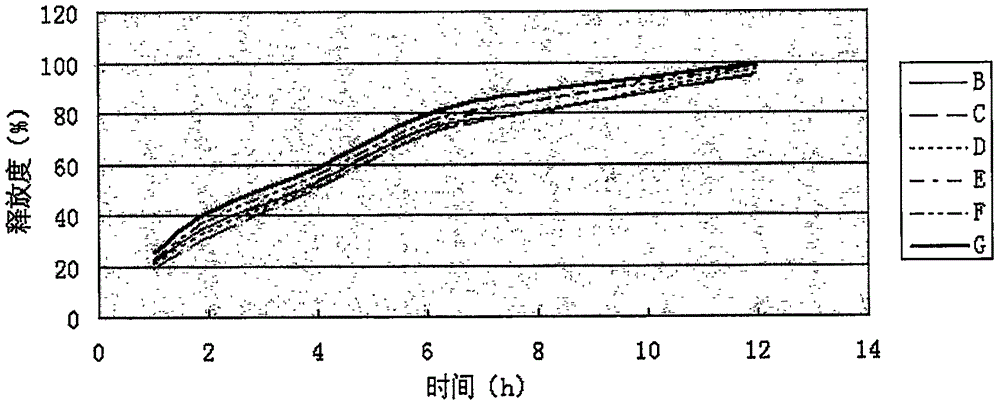
Image
Examples
Embodiment 1
[0027] A kind of dabrafenib mesylate sustained-release tablet, prepared according to the following steps:
[0028] Chip composition:
[0029]
[0030]
[0031] Coating composition:
[0032]
[0033] Preparation process:
[0034] (1) Take dabrafenib mesylate, hypromellose, lactose, sodium alginate, pregelatinized starch, and microcrystalline cellulose in the prescribed amount and mix them into fine powder (all pass through a No. 5 sieve and The amount of the No. 6 sieve that can pass must not be less than 95% of the total amount), sieved;
[0035] (2) Add the prescribed amount of ethanol solution to the above mixed powder, mix and granulate (extrude and granulate through a 24-mesh sieve), put the prepared wet granules in a hot air oven, set the temperature at 40-60°C, and dry When the moisture content of the granules is ≤3%, the whole granules (through a 18-mesh sieve) are set aside;
[0036] (3) the magnesium stearate of recipe quantity is pulverized and crossed 1...
Embodiment 2
[0075] A kind of dabrafenib mesylate sustained-release tablet, prepared according to the following steps:
[0076] Chip composition:
[0077]
[0078] Coating composition:
[0079]
[0080] Preparation process: prepared according to the preparation process of Example 1.
[0081] (1) Determination of release rate
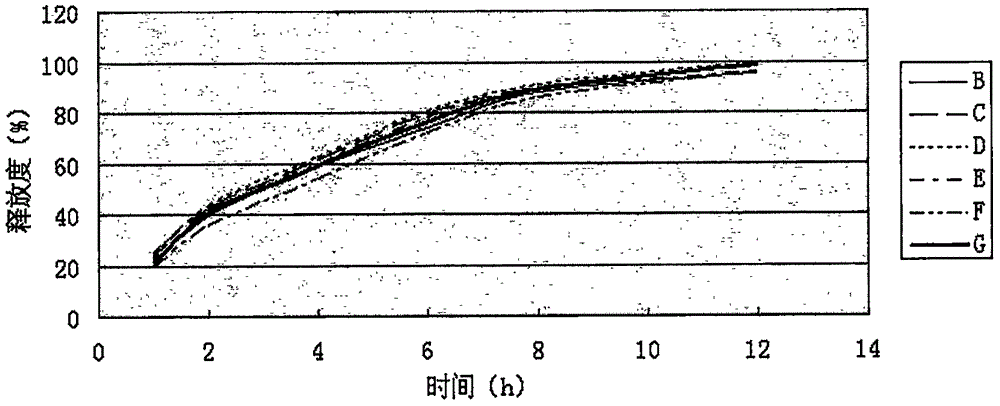
[0082] Measure according to embodiment 1 release degree measuring method, the measurement result of its release degree is shown in Table 2, figure 2 (done six samples to measure).
[0083] Table 2 Sustained-release tablet sample release rate (%) of the present invention
[0084]
[0085]
[0086] test results:
[0087] Appearance: Film-coated tablet with smooth surface.
[0088] Release: Dabrafenib Mesylate Sustained-release Tablets The main drug, Dabrafenib Mesylate, is released slowly, which can meet the requirements of sustained-release tablets.
[0089] (2) A kind of dabrafenib mesylate sustained-release tablet stability experiment of the pres...
Embodiment 3
[0110] A kind of dabrafenib mesylate sustained-release tablet, prepared according to the following steps:
[0111] Chip composition
[0112]
[0113]
[0114] Coating composition:
[0115]
[0116] Preparation process: prepared according to the preparation process of Example 1.
[0117] (1) Determination of release rate
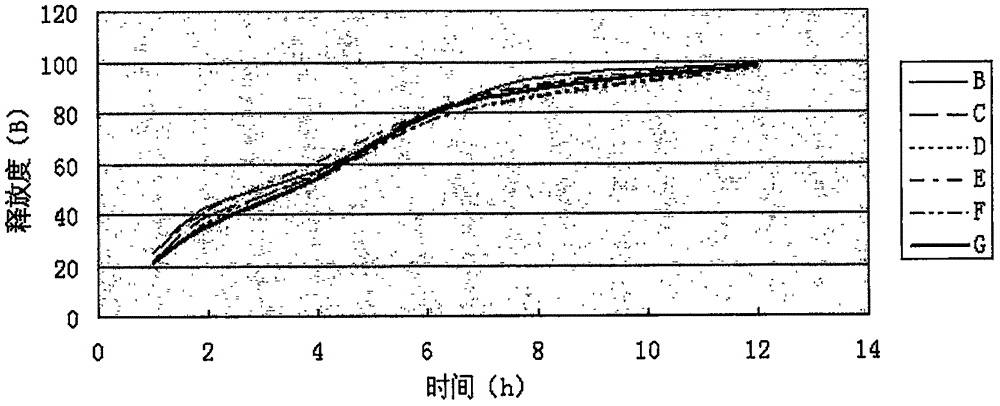
[0118] Measure according to embodiment 1 release degree measuring method, the measurement result of its release degree is shown in Table 3, image 3 (done six samples to measure).
[0119] Table 3 Sustained-release tablet sample release rate (%) of the present invention
[0120]
[0121] test results:
[0122] Appearance: Film-coated tablet with smooth surface.
[0123] Release: Dabrafenib Mesylate Sustained-release Tablets The main drug, Dabrafenib Mesylate, is released slowly, which can meet the requirements of sustained-release tablets.
[0124] (2) A kind of dabrafenib mesylate sustained-release tablet stability experiment of the presen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com