Package of sensitive articles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
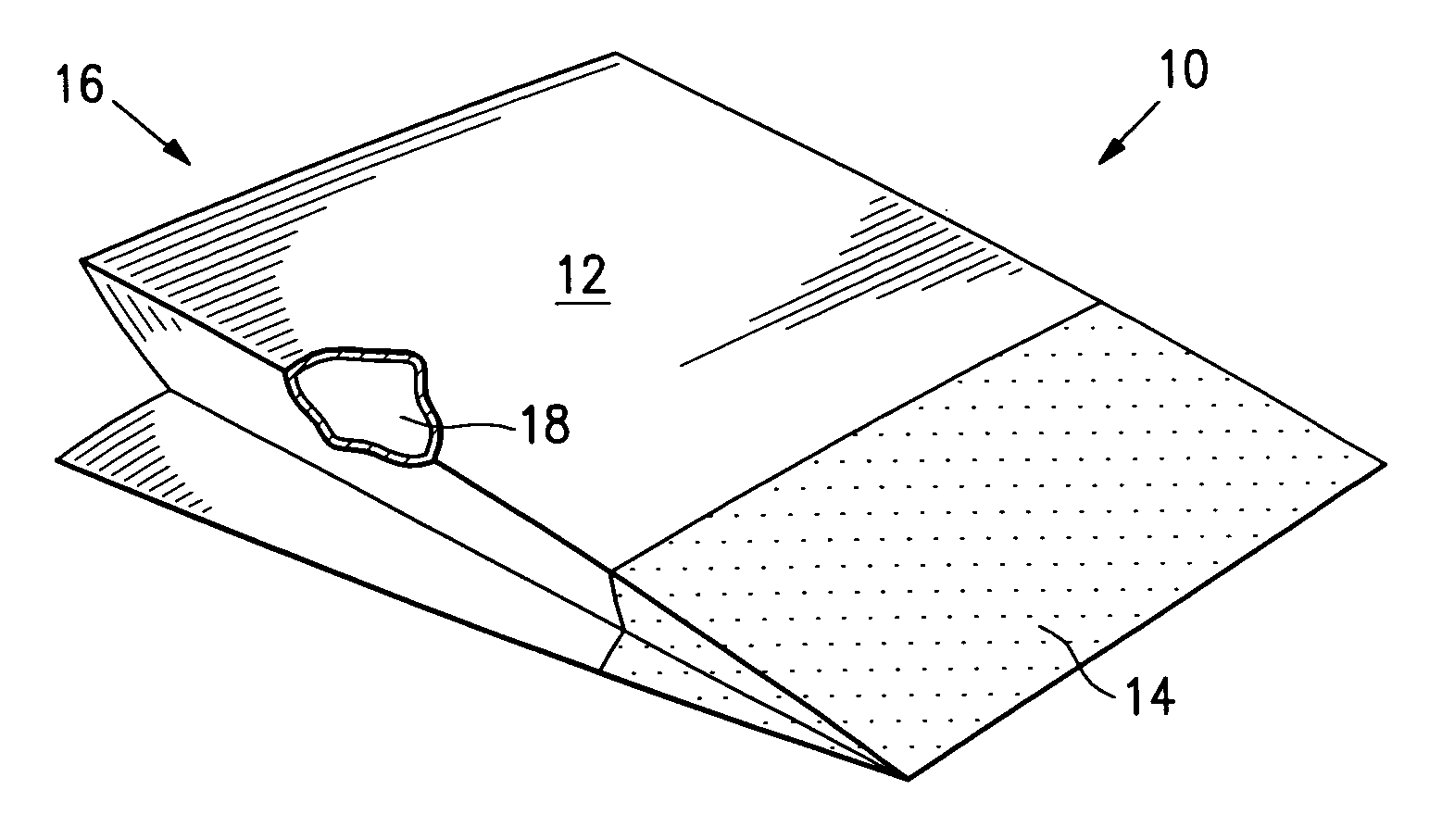
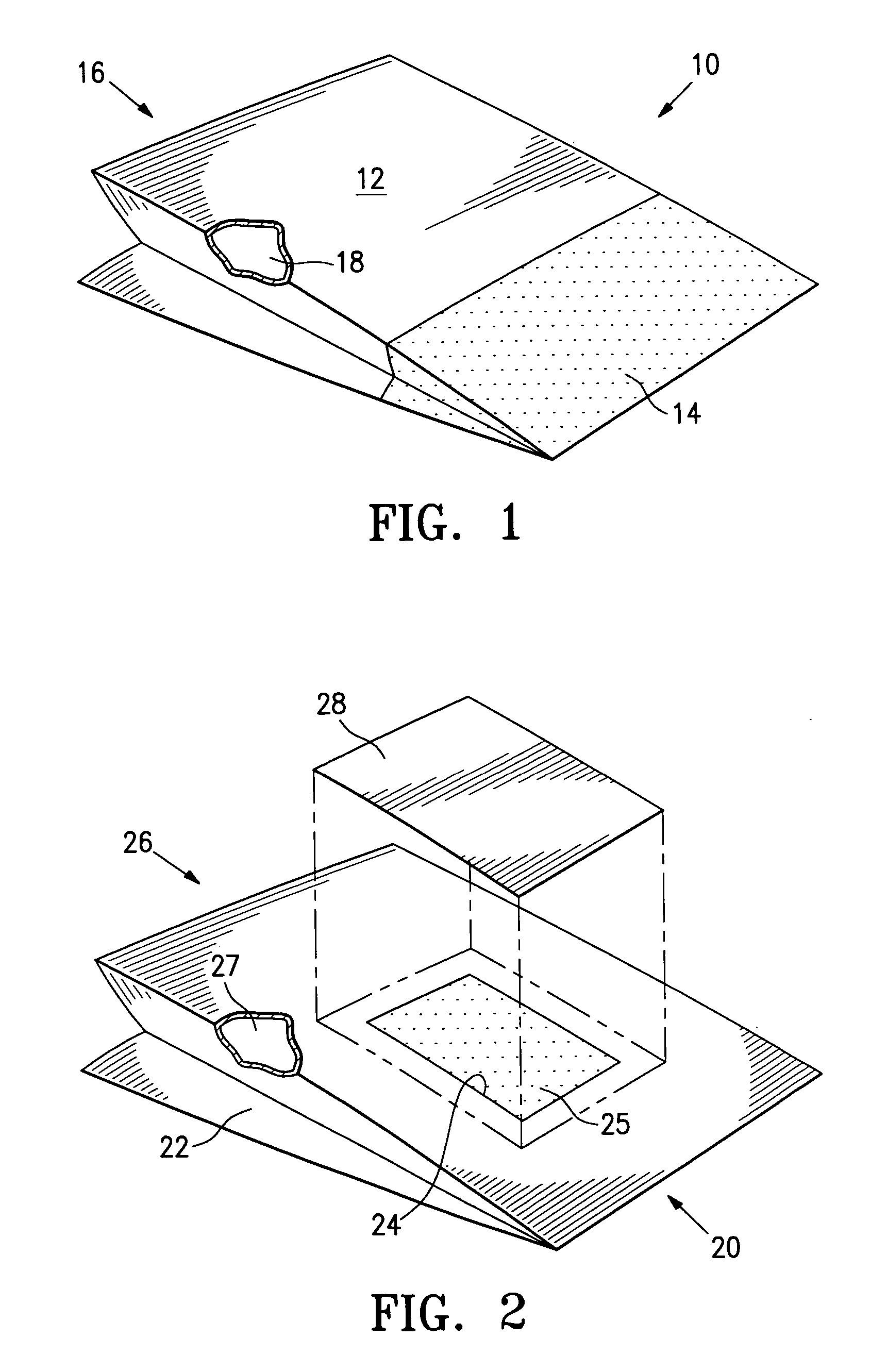
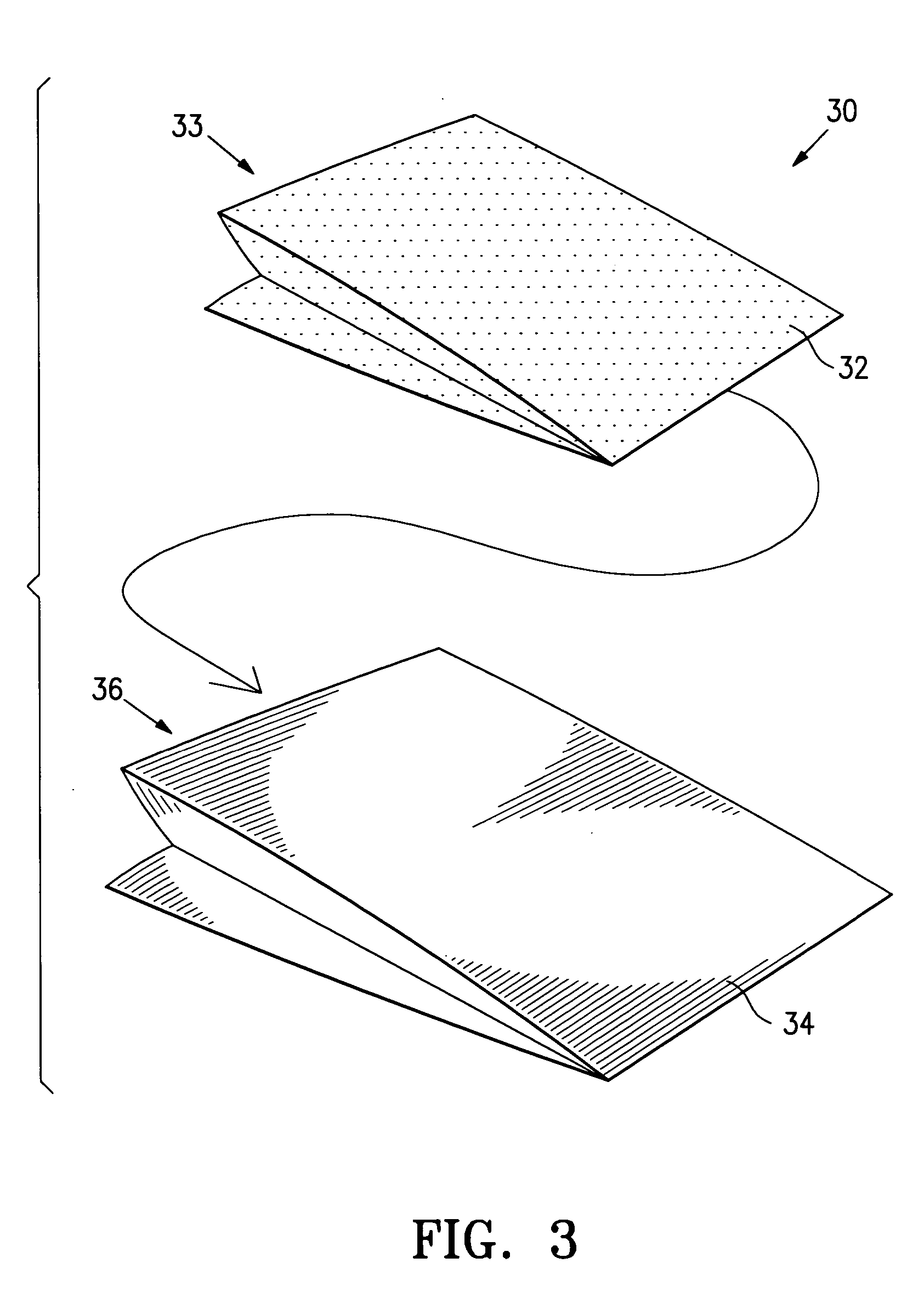
[0048] A drug eluting stent mounted onto the balloon of a stent delivery catheter (a drug eluting stent system) is packaged in a foil pouch that has a permeable portion or patch formed of Tyvek®. The pouch is subjected to a vacuum within the inner chamber with a surrounding atmosphere of EtO gas at temperatures of 60° C. to sterilize the drug eluting stent and delivery catheter. After sterilization, the pouch is resealed with the stent and delivery system within the post seal area and the Tyvek patch outside the post-seal area. The foil pouch minimizes the light exposure as well the oxygen and moisture exposure to the sterilized contents of the container.
example 2
[0049] A drug eluting stent mounted onto the balloon of a stent delivery catheter is packaged in a Tyvek pouch along with ferrous oxide as an oxygen absorber and desiccant. The pouch is closed, subjected to ethylene oxide gas which permeates the pouch. After sterilization, the Tyvek pouch is evacuated and purged with nitrogen and air to remove essentially all EtO and then placed inside a gas impermeable foil pouch. The foil pouch is evacuated by vacuum to remove the remaining air and nitrogen gas, and then finally sealed. The foil pouch minimizes the exposure of the sterilized product within the Tyvek pouch to light and to oxygen and moisture, thus providing a long shelf life.
example 3
[0050] A drug eluting stent crimped onto a PTCA catheter or a drug eluting stent system is packaged on Tyvek pouch along with ferrous oxide oxygen absorbers and desiccant. The pouch is sterilized by ethylene oxide gas with sterilization temperatures of about 55° C. After sterilization, the sterilized Tyvek pouch with the stent system sealed inside is inserted inside a foil pouch. The foil pouch is sealed using a vacuum sealer that first purge the bag with nitrogen gas which replaces the air inside the foil pouch and that finally seal the foil pouch. This minimizes the light exposure as well as limit oxygen and moisture exposure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com