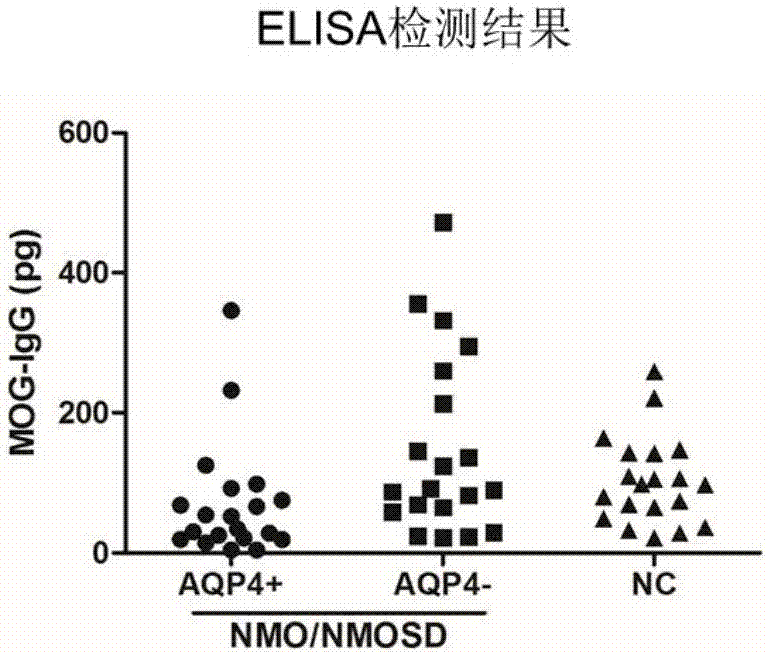
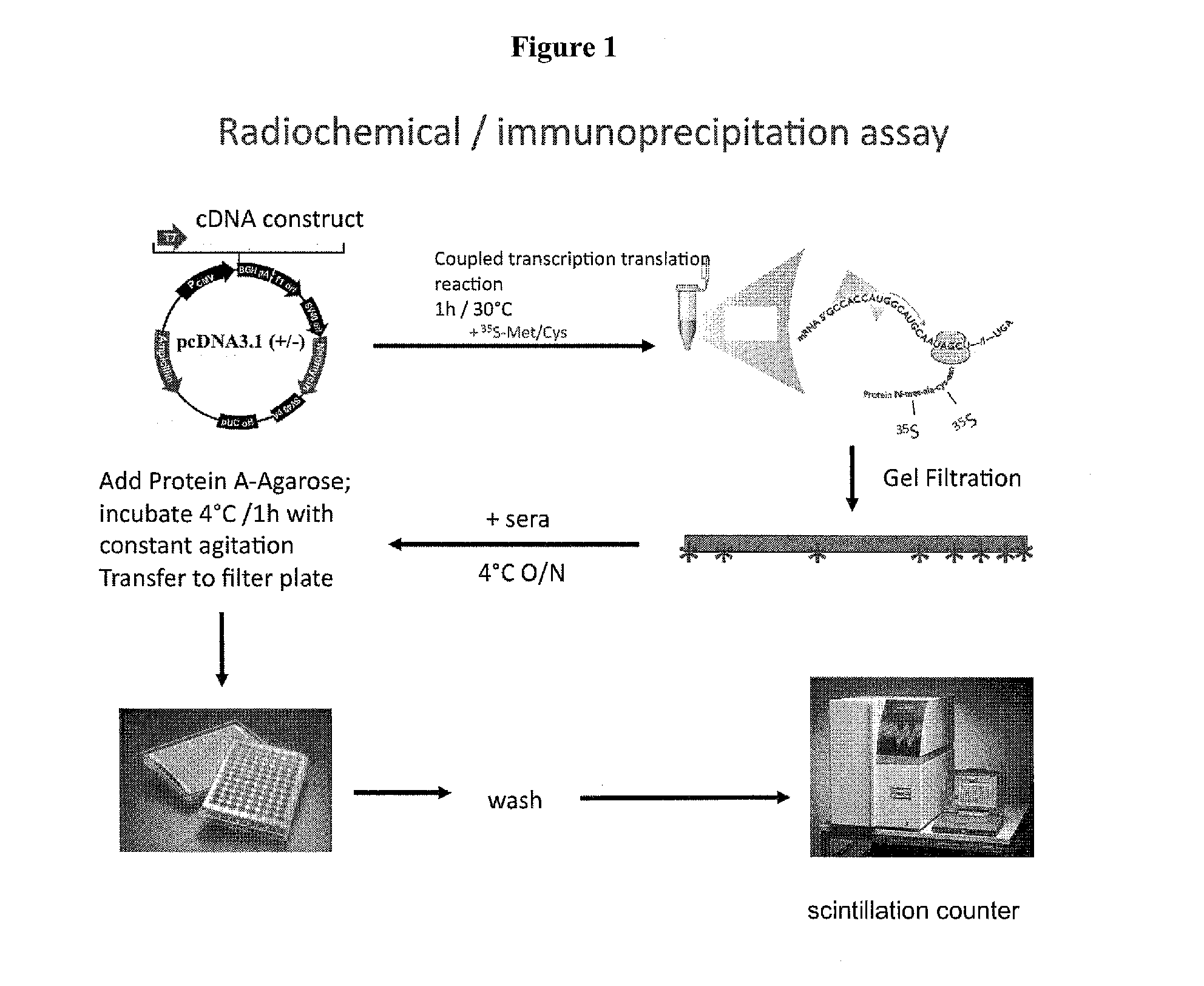
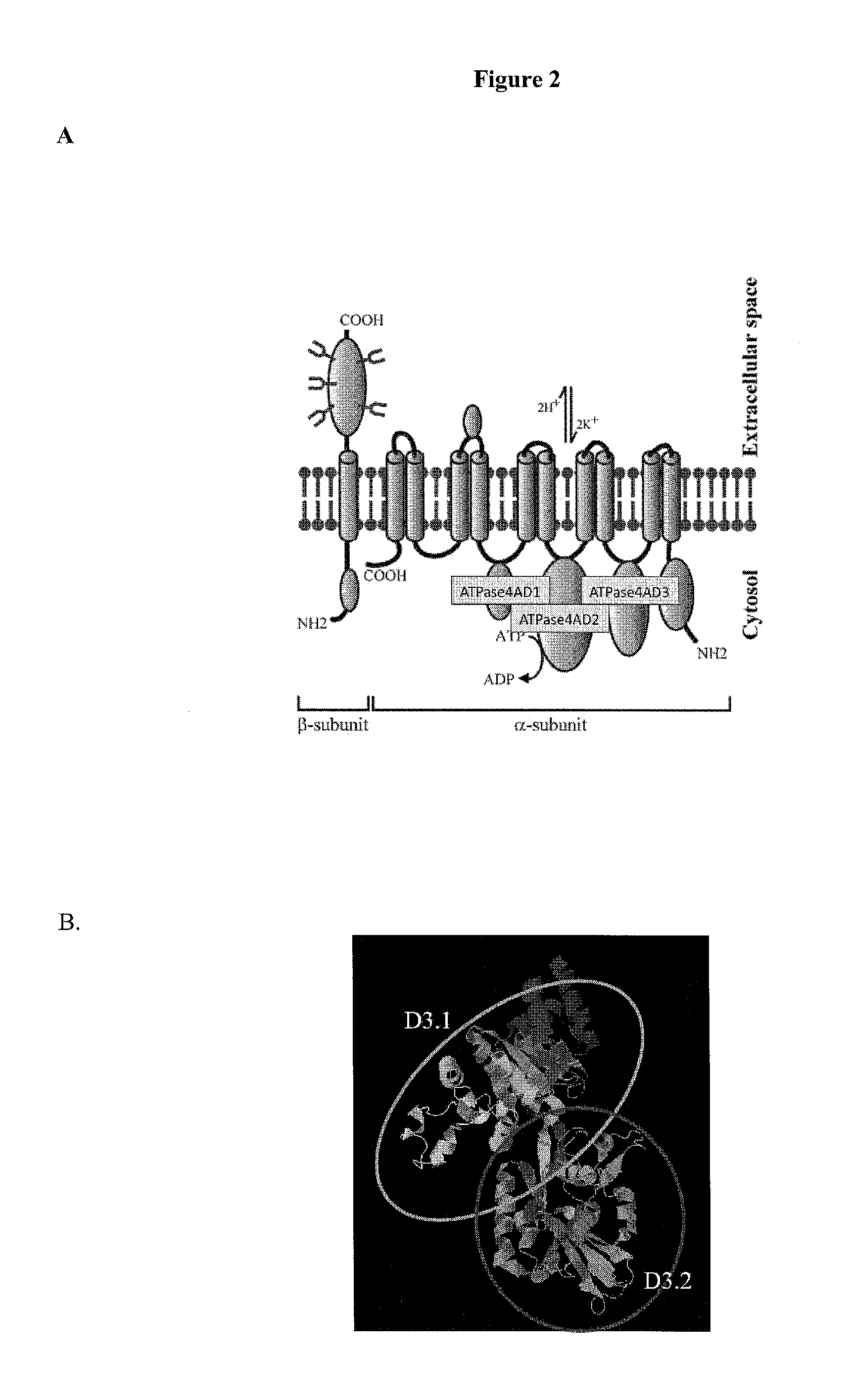
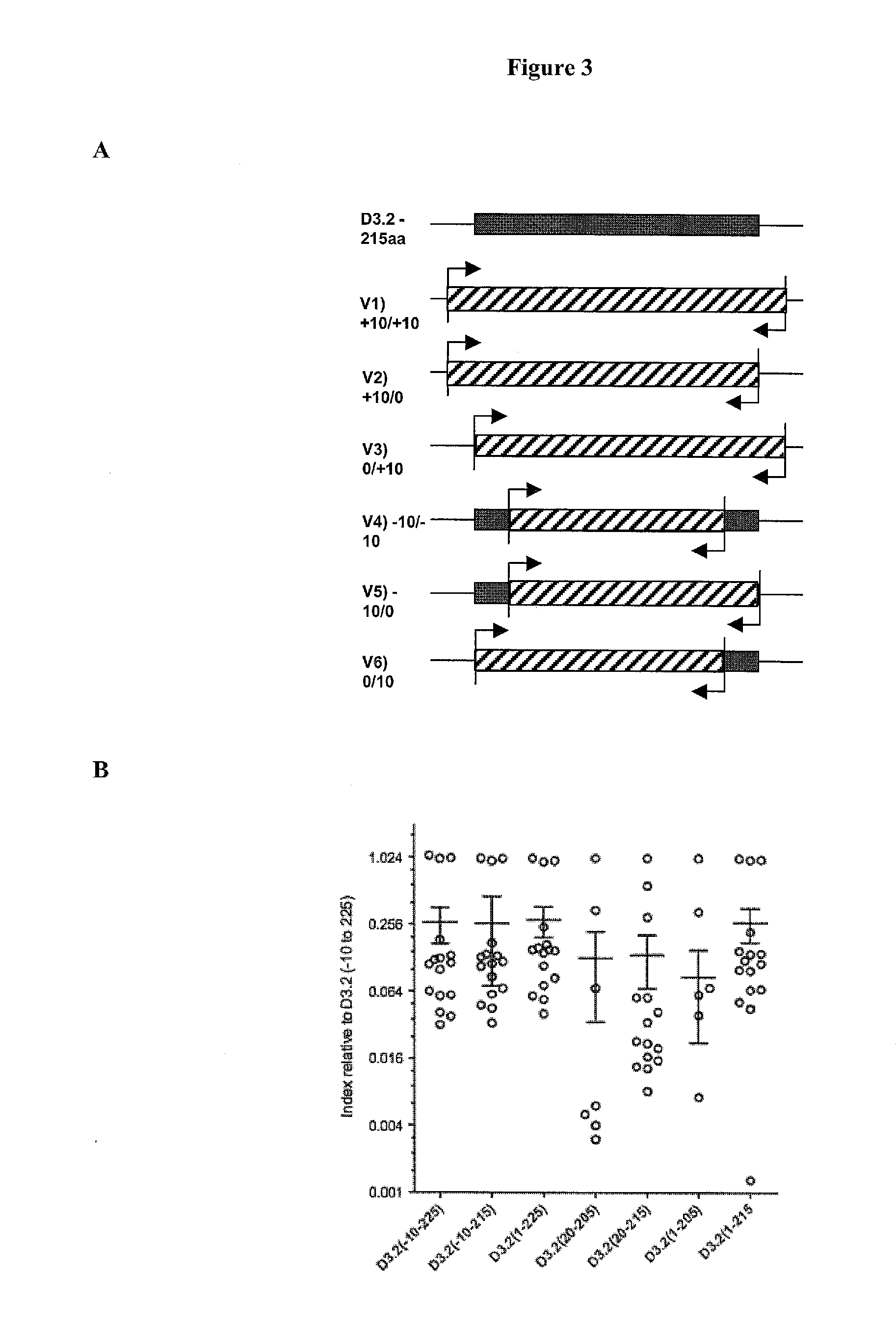
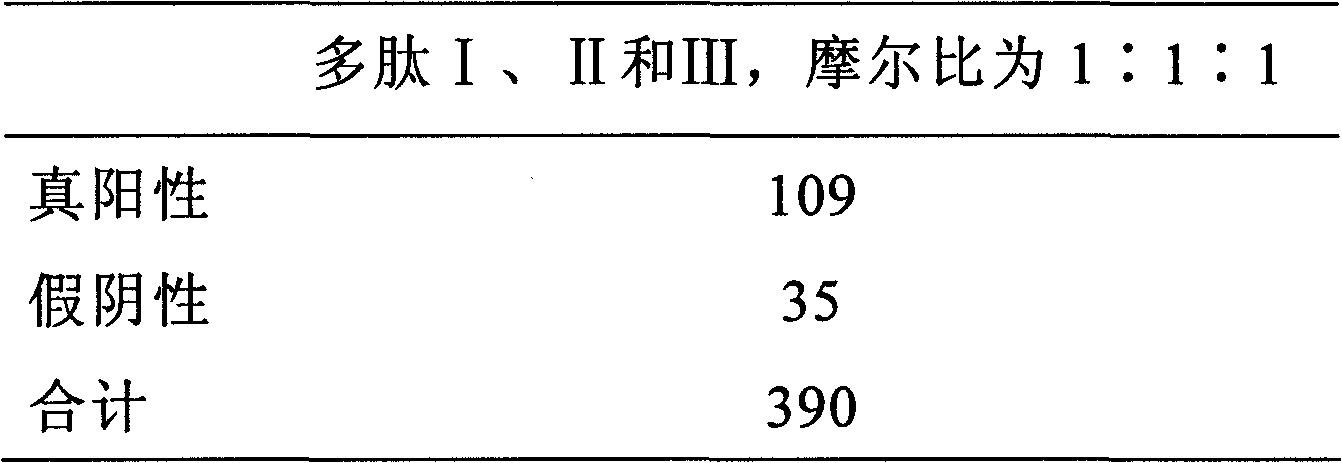
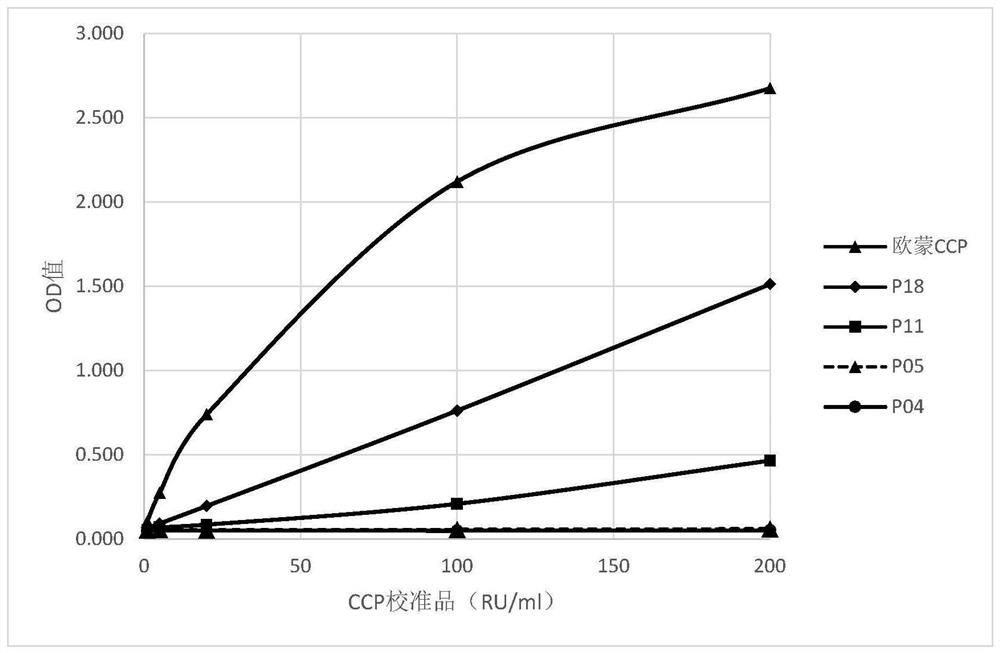
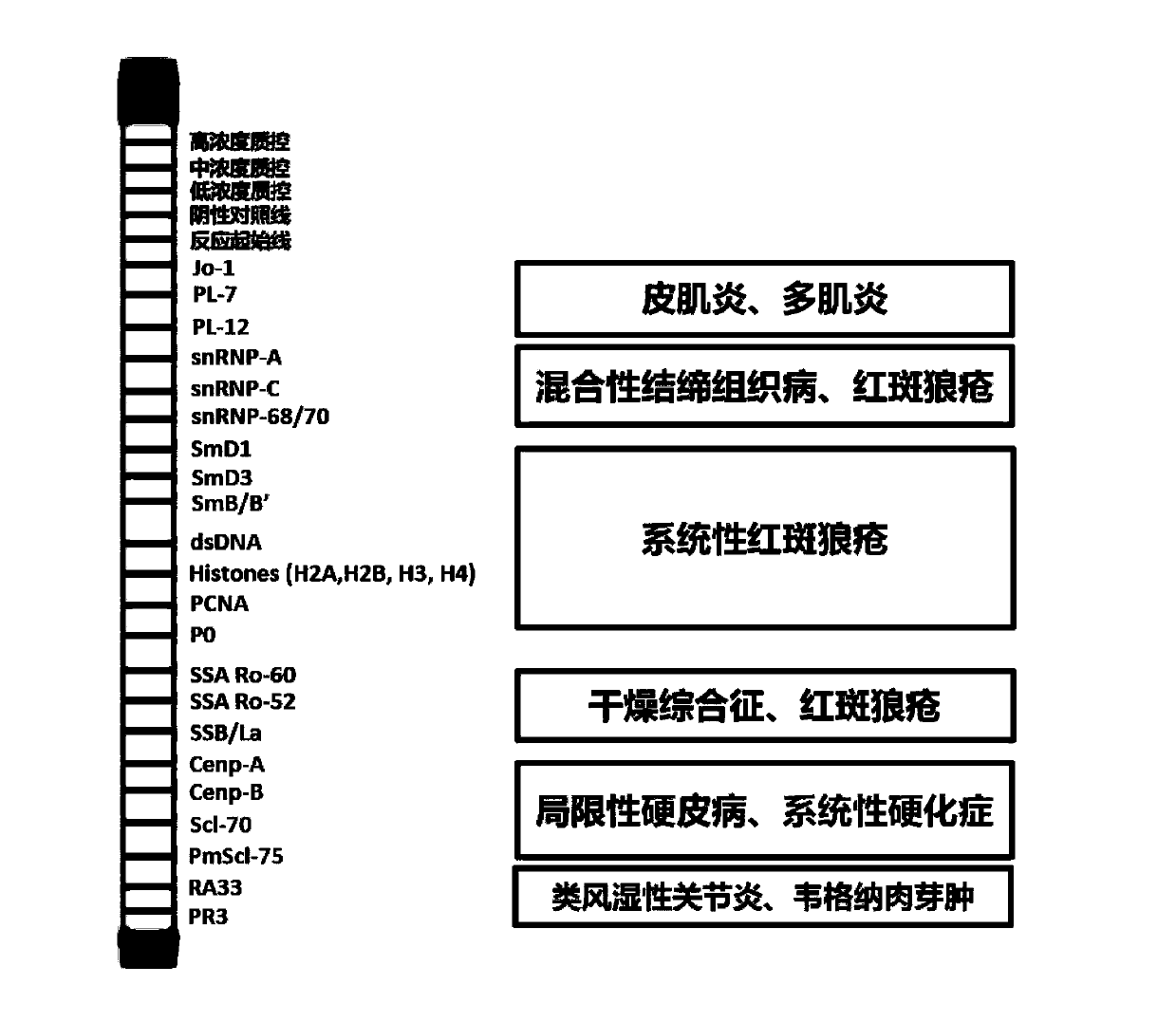
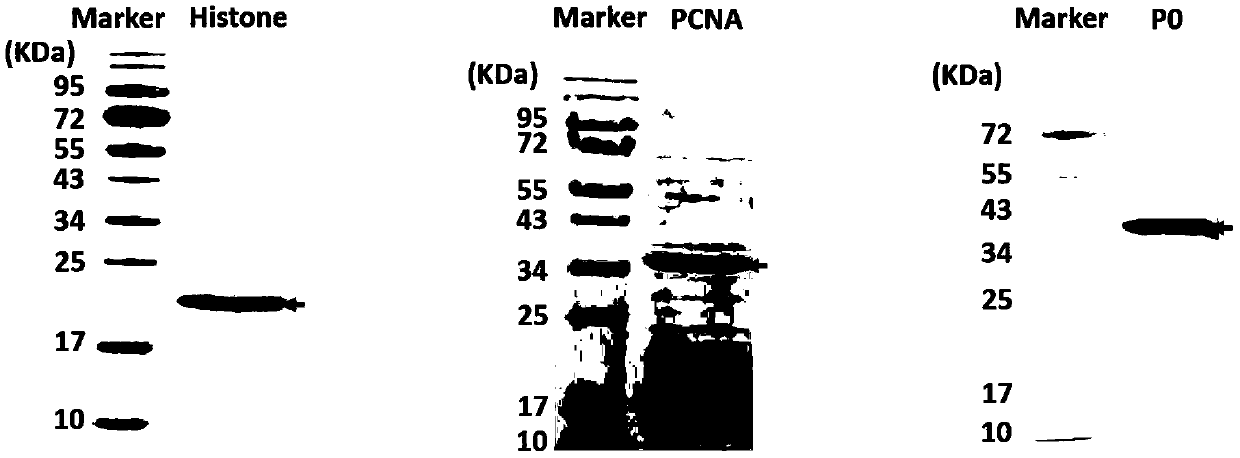
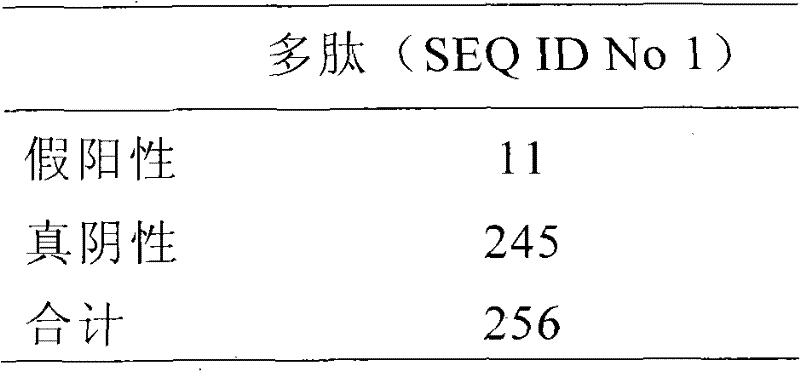
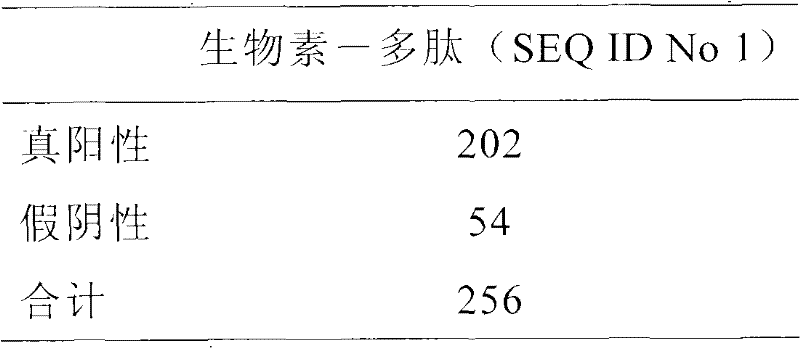
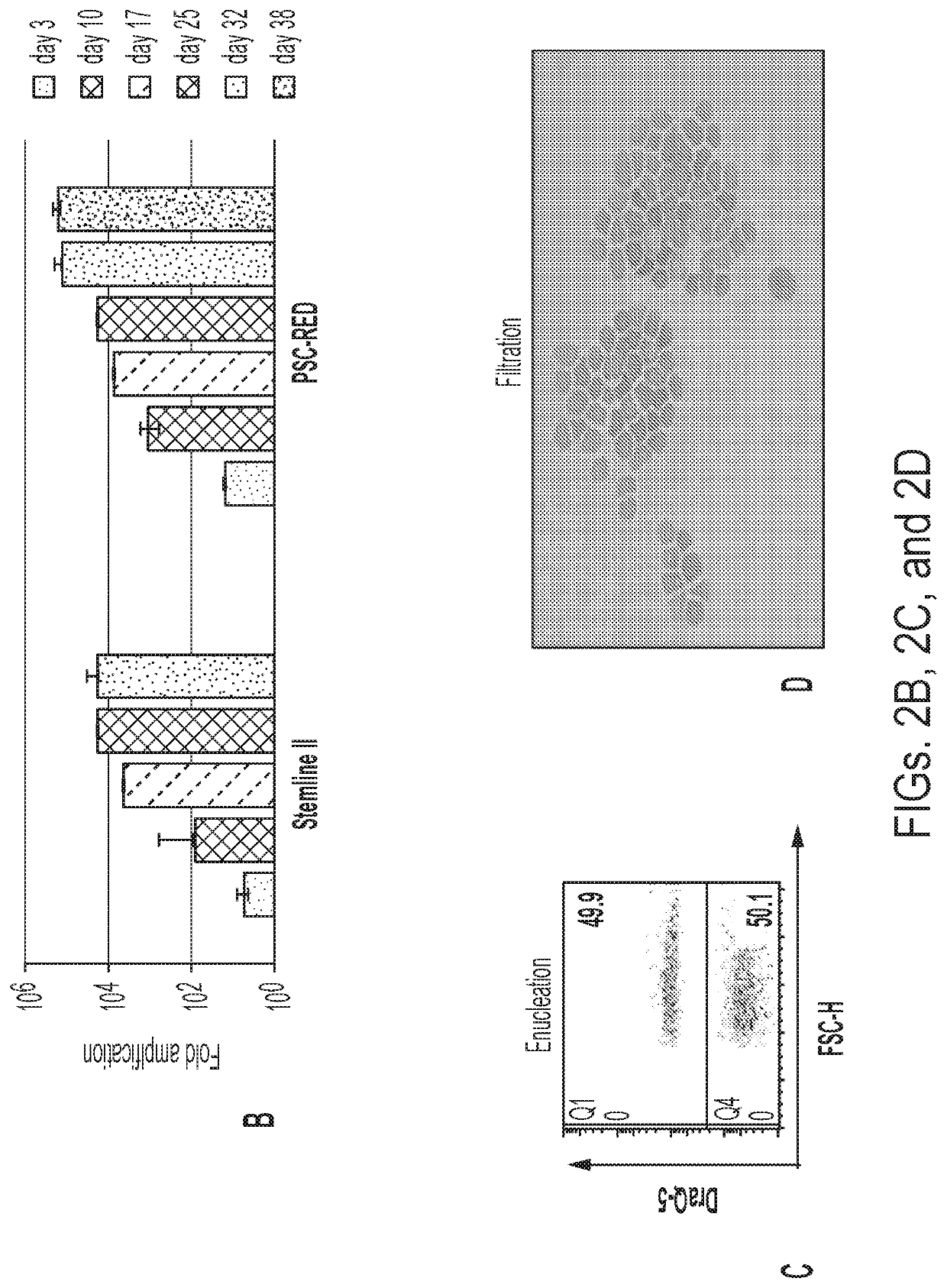
Patents
Literature
43 results about "Autoimmune antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Autoimmune antibodies are groups of immune proteins that can be damaging to the human body, as they target tissues and organs and cause deterioration.
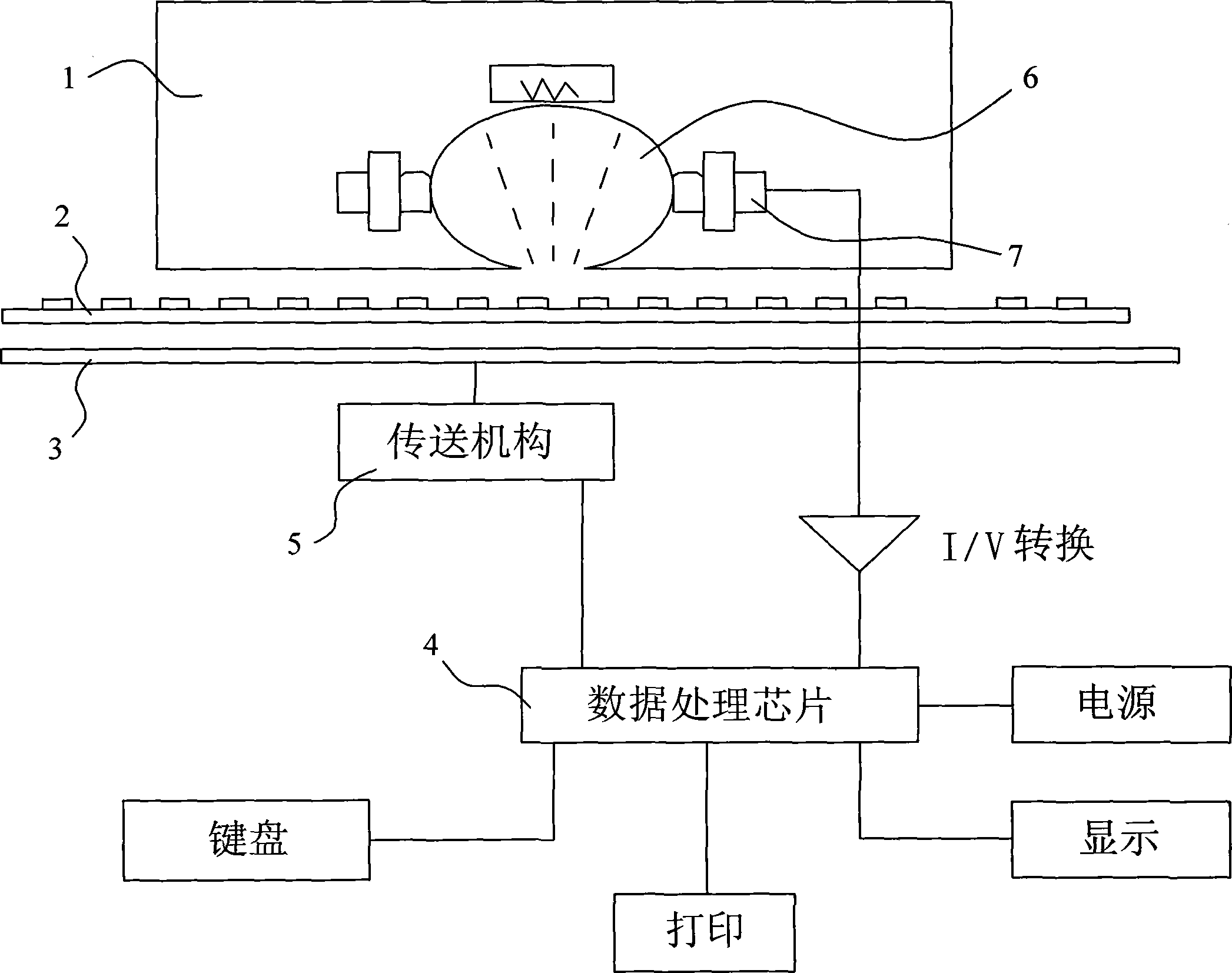
Albumen chip for detecting autoimmunity antibody of diabetes, as well as preparation and detection method
InactiveCN1415964AHigh clinical practical valueReduce testing costsBiological testingFluorescence/phosphorescenceDiabetes mellitusFluorescence
This invention relates to preparation and detect method of detection protein chip of diabetes autoimmune antibody. The scheme is as follows: a protein chip is a thin glass chip set with multiple reaction tanks on top surface of the said shell and a detection microarray in each of the reaction tank, every micro-array is composed of three different kinds, same size protein micro-solid phase detection chips of GAP, PTP and Insulin. The preparation method is to prepare protein micro-solid phase detection chips to be assembled and the detecting method is to apply indirect fluorescent method for detection.
Owner:XIAN BESTEDIT BIOMEDICAL SERVICES
Autoantibodies utilized as carrier agents for pharmaceutical compounds used in tumor imaging and cancer treatment
ActiveUS20050147603A1Non-immunogenicAnimal cellsRadioactive preparation carriersAbnormal tissue growthImaging agent
This invention describes a method whereby human autoimmune antibodies are used as carrier compounds to deliver imaging agents and pharmaceutical drugs to the tumor in the human patient. These autoantibodies have the propensity to localize in necrotic areas of tumors but not in healthy normal tissues. By combining various pharmaceutical agents with these carrier proteins it is possible to localize these agents within the necrotic areas of tumors in cancer patients. The carrier proteins may be combined with a variety of imaging agents for detection and diagnosis of tumors, and / or with a variety of radioactive or cytotoxic compounds for cancer treatment.
Owner:SMITH HENRY JOHN +1
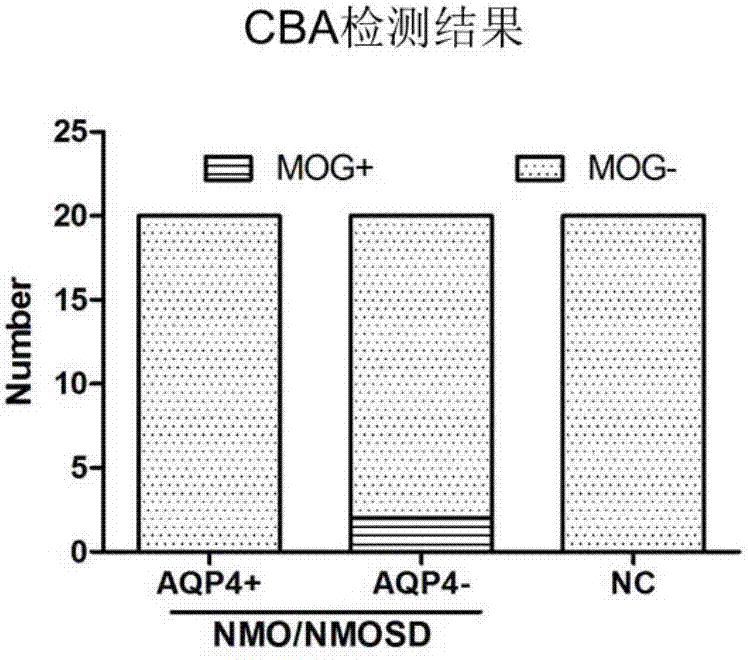
Preparation of cell growing slide assembly of CBA (cytometric bead array) kit for detecting MOG-IgG, CBA detection kit and application of CBA detection kit
InactiveCN107299111AGood repeatabilityStrong specificityCell dissociation methodsBiological material analysisCell strainBead array
The present invention relates to the preparation of a cell slide assembly for a CBA kit for detecting MOG-IgG, the CBA detection kit and its application. The preparation method of the cell slide assembly is as follows: S1: construction of MOG plasmid; S2: overexpression of MOG gene Lentiviral packaging; S3: Construction of a cell line (MOG-293T cell line) overexpressing the MOG gene; S4: Preparation of cell slide components. The kit includes cell slide assembly, anti-human IgG-FITC secondary antibody, blocking solution, PBS and mounting medium; the kit can be used for detection of MOG autoimmune IgG antibody in clinical samples. The kit of the invention has the advantages of simple operation, strong detection specificity, high sensitivity, good repeatability of experimental results, can be popularized and applied, and can be used for detection of MOG autoimmune IgG antibody in clinical samples.
Owner:GENERAL HOSPITAL OF PLA
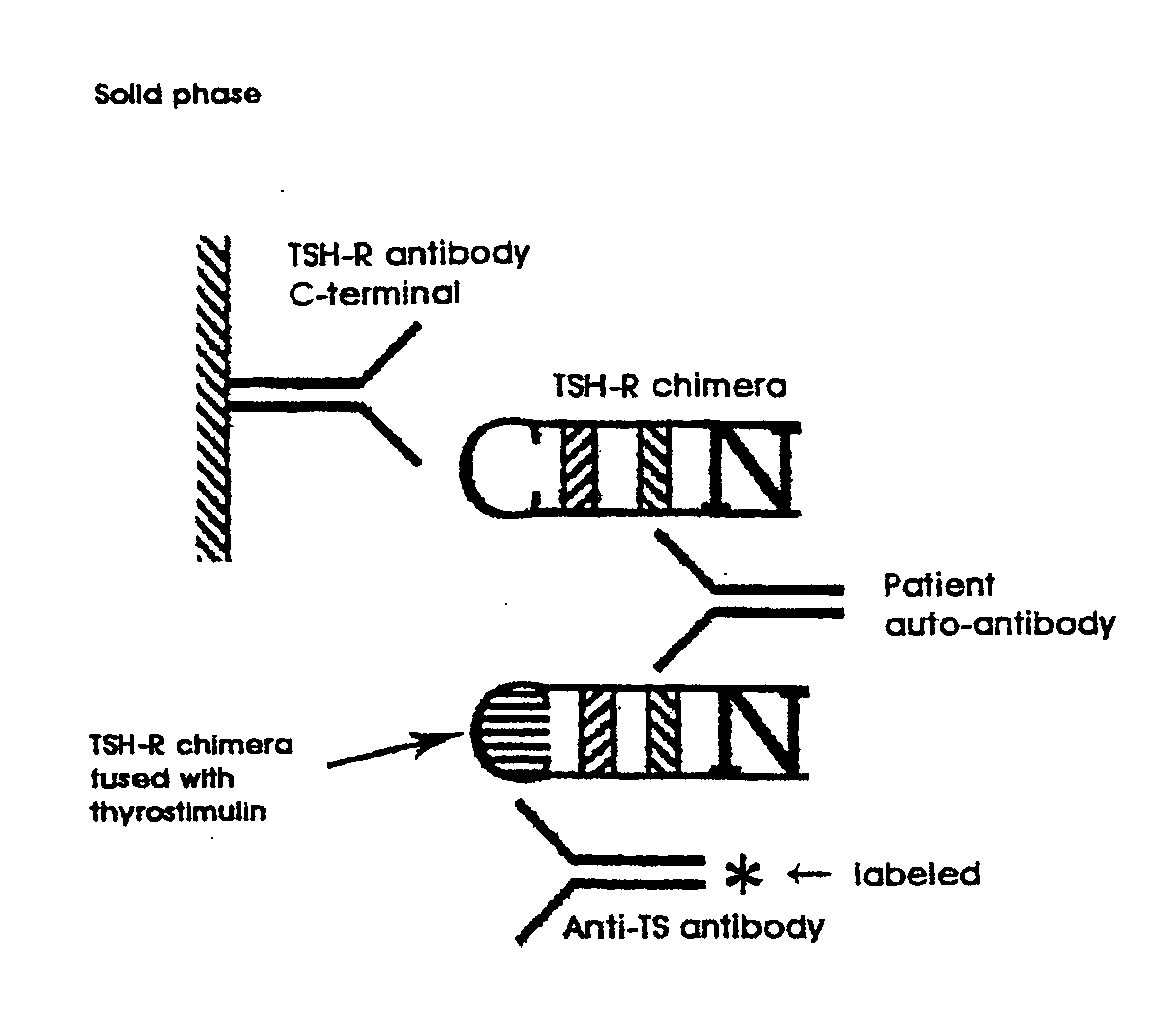
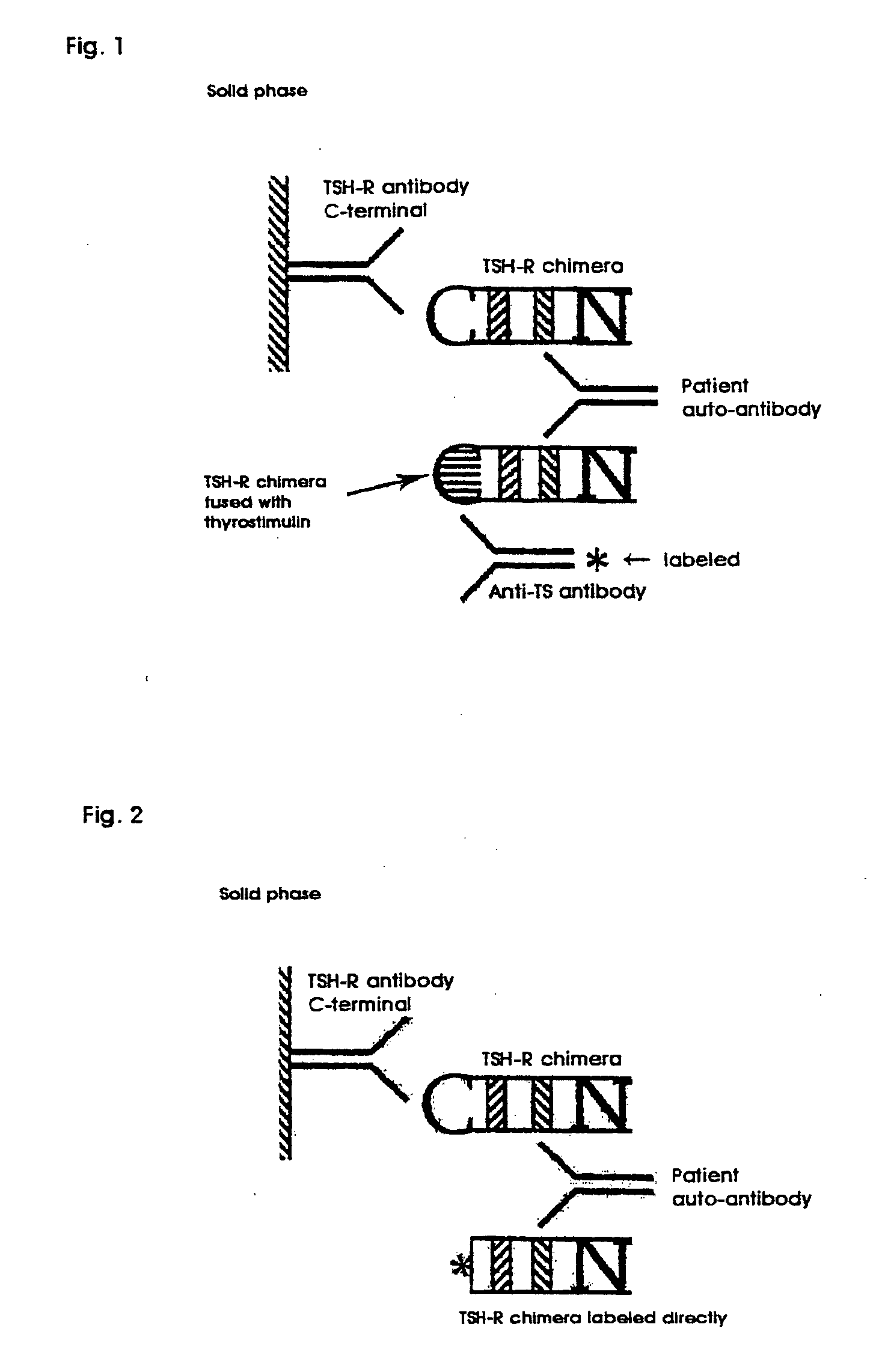
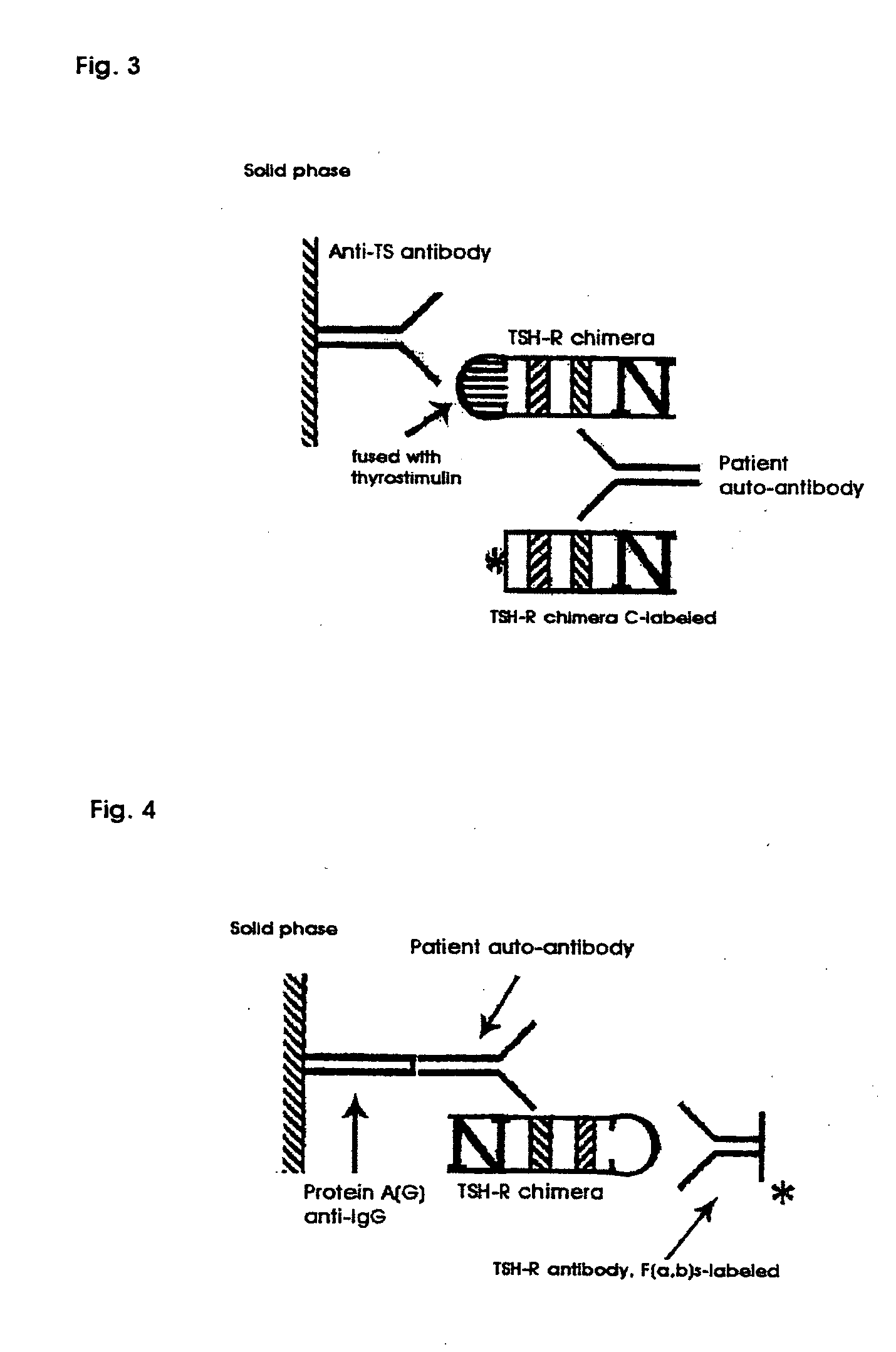
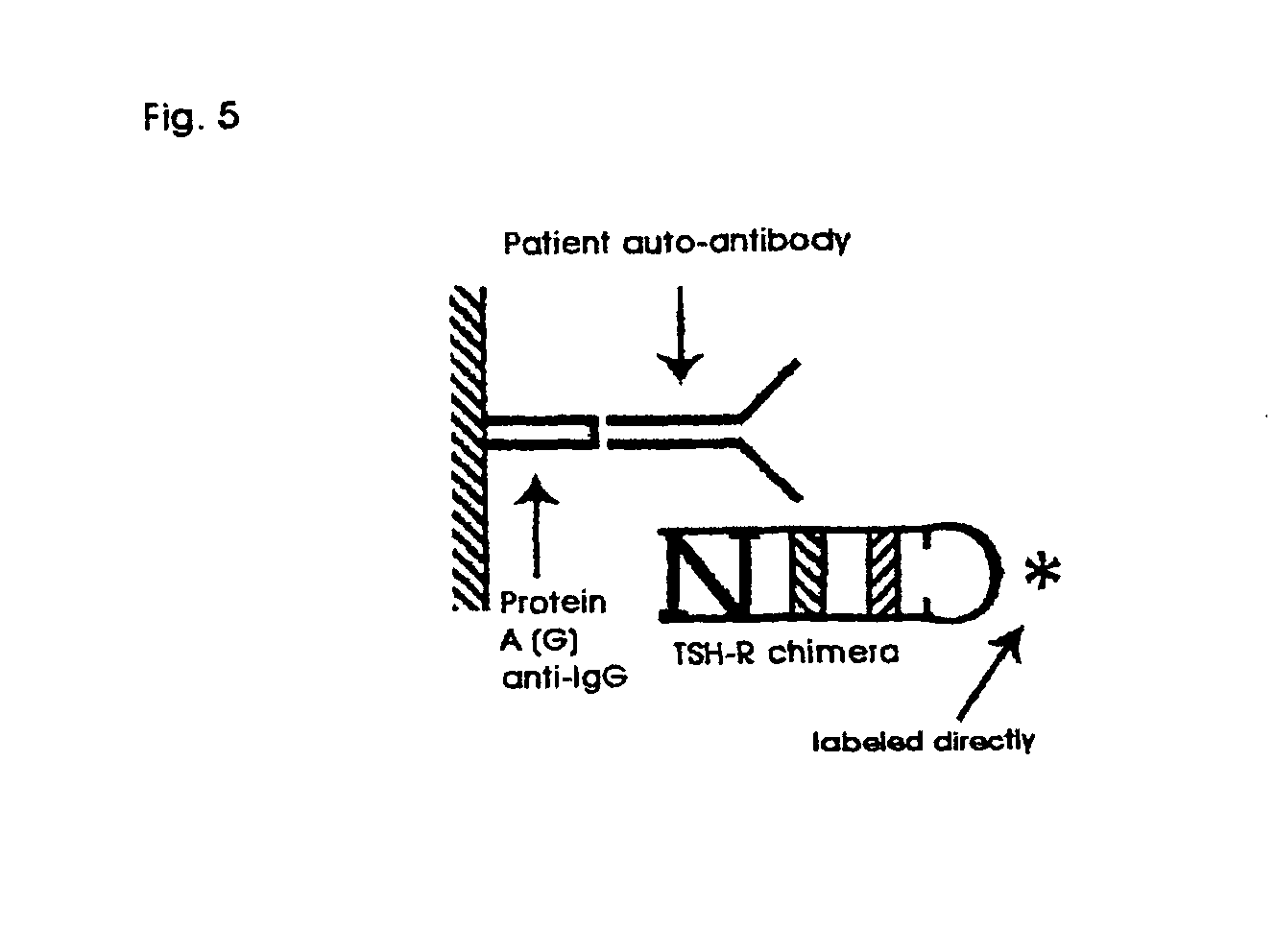
Innovativer TSH-R-Ab-Kit
ActiveUS20090325310A1Improve accuracyImprove expressivenessHydrolasesPeptide preparation methodsWild typeImmunogenic peptide
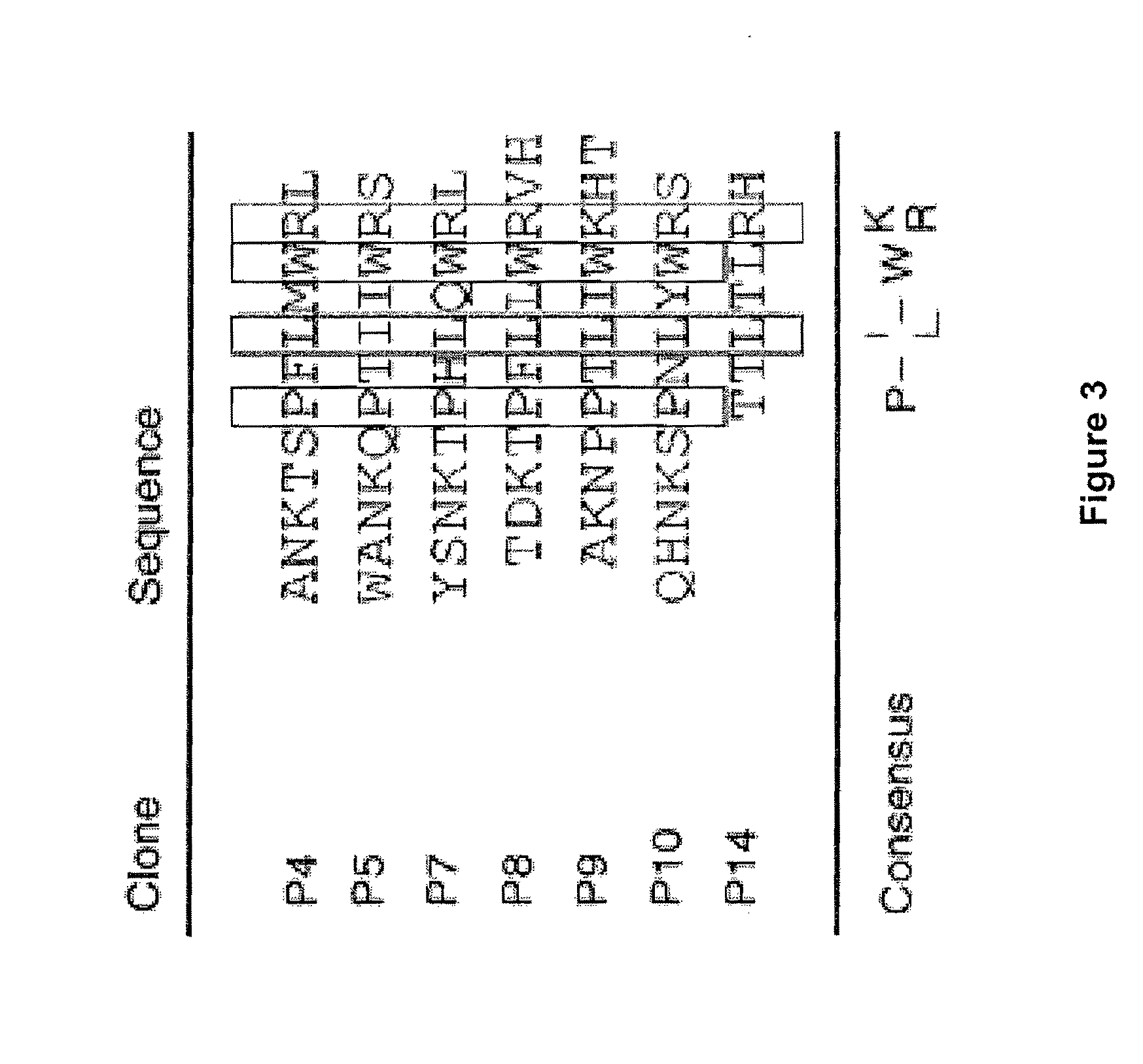
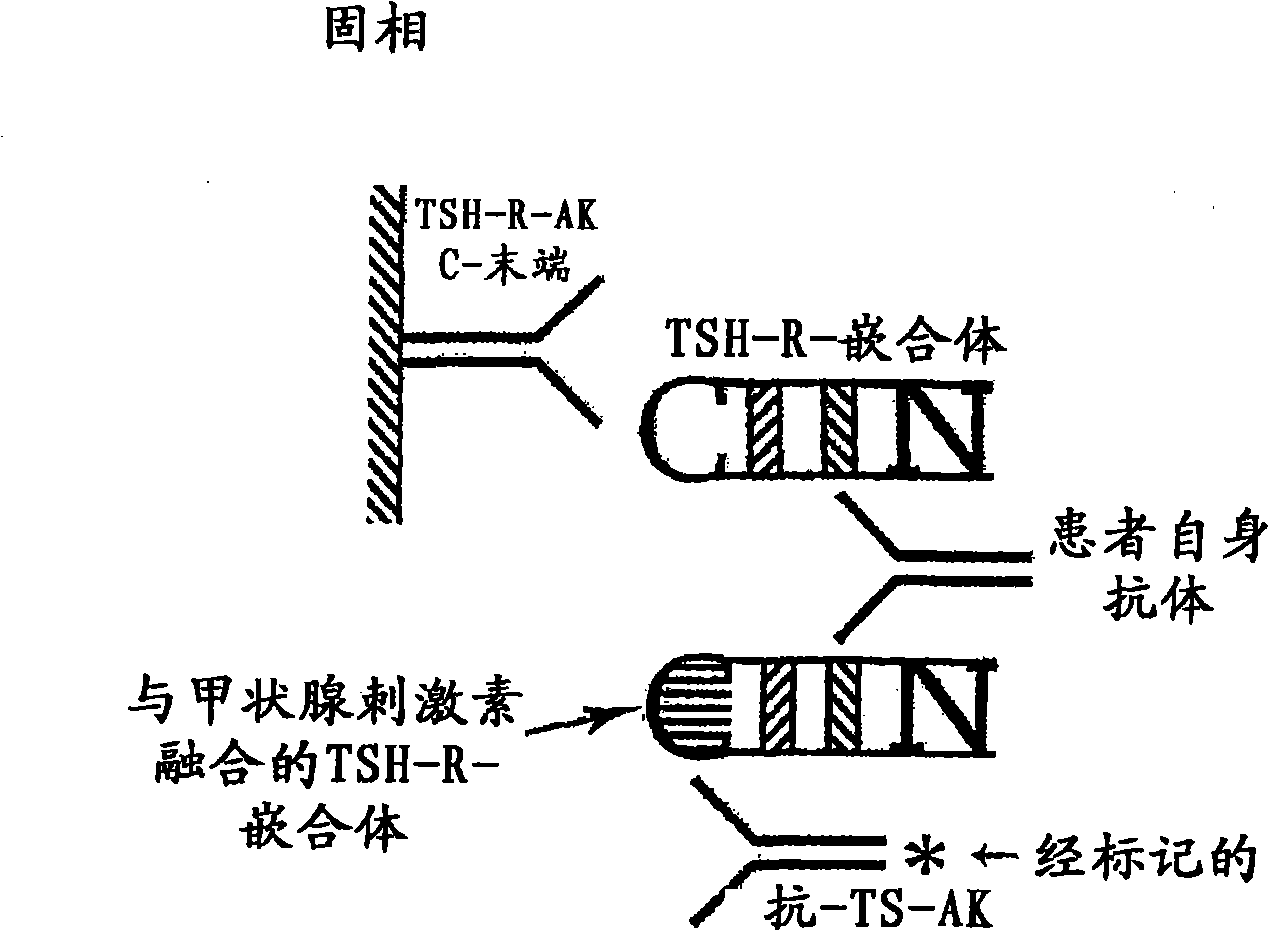
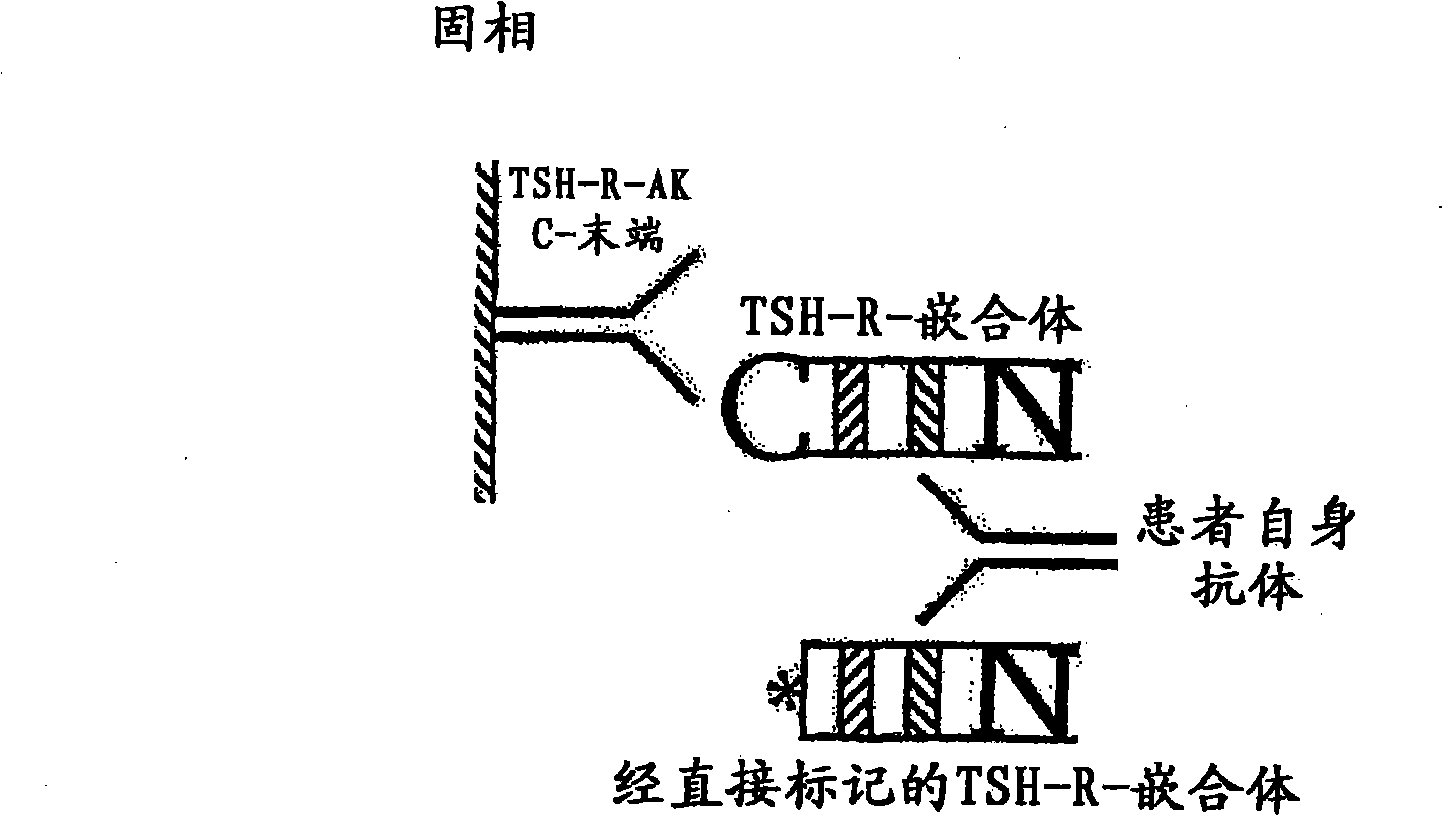
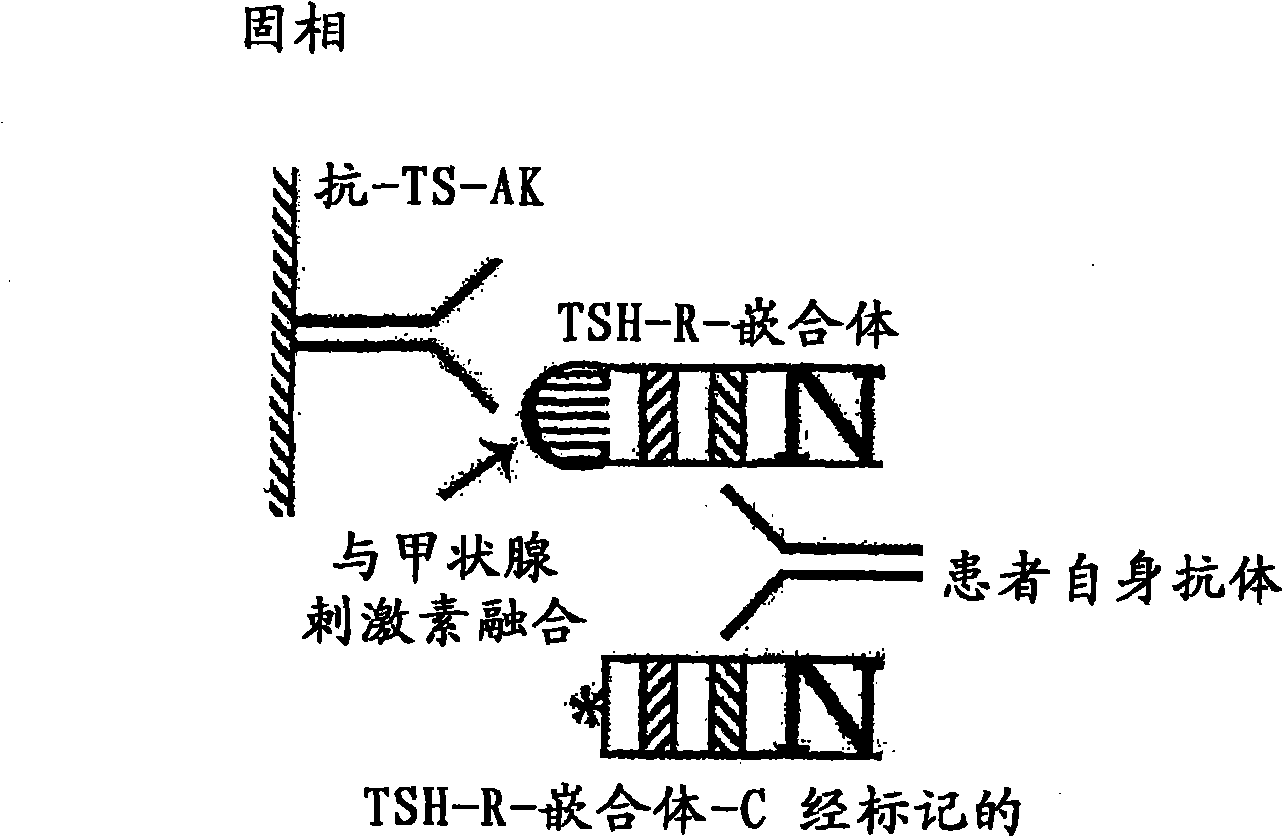
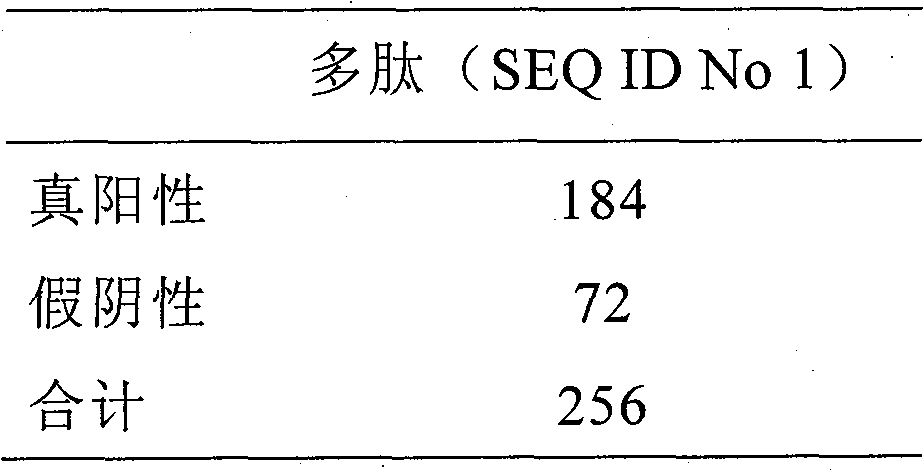
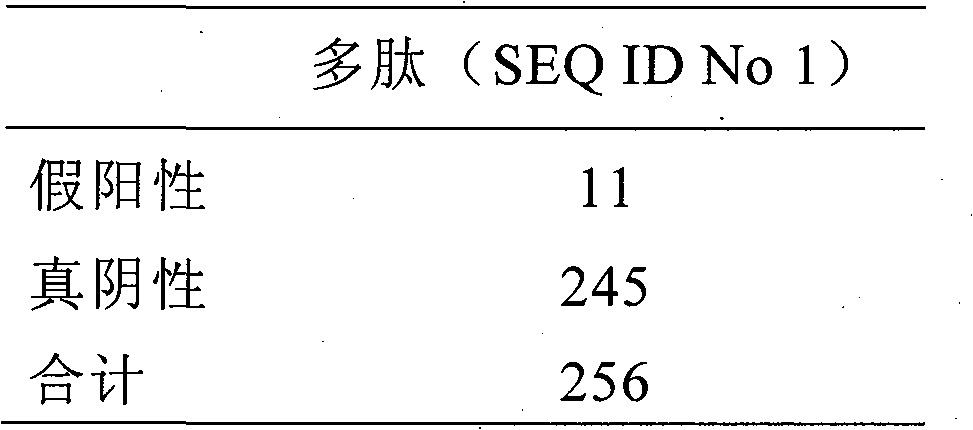
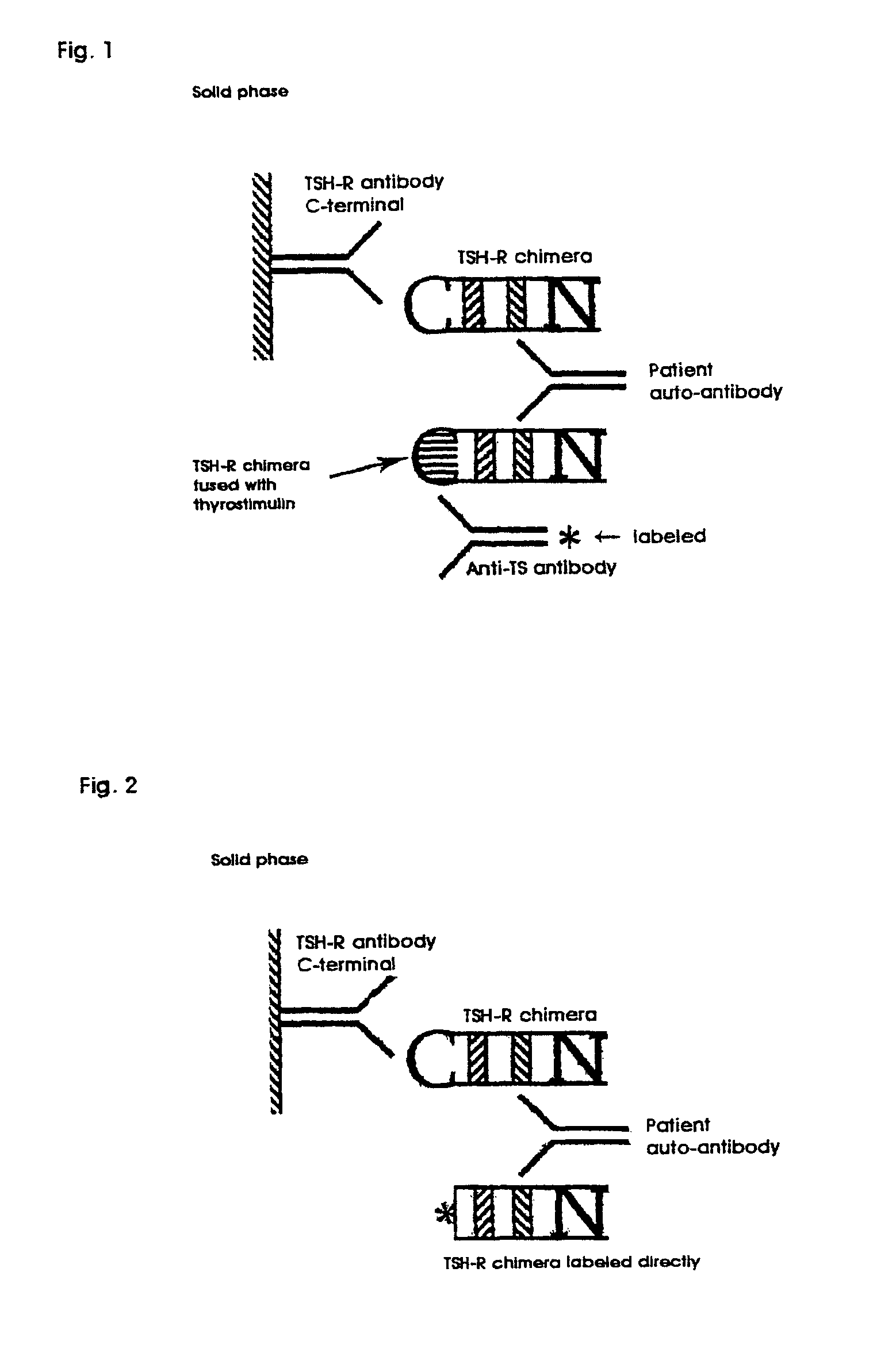
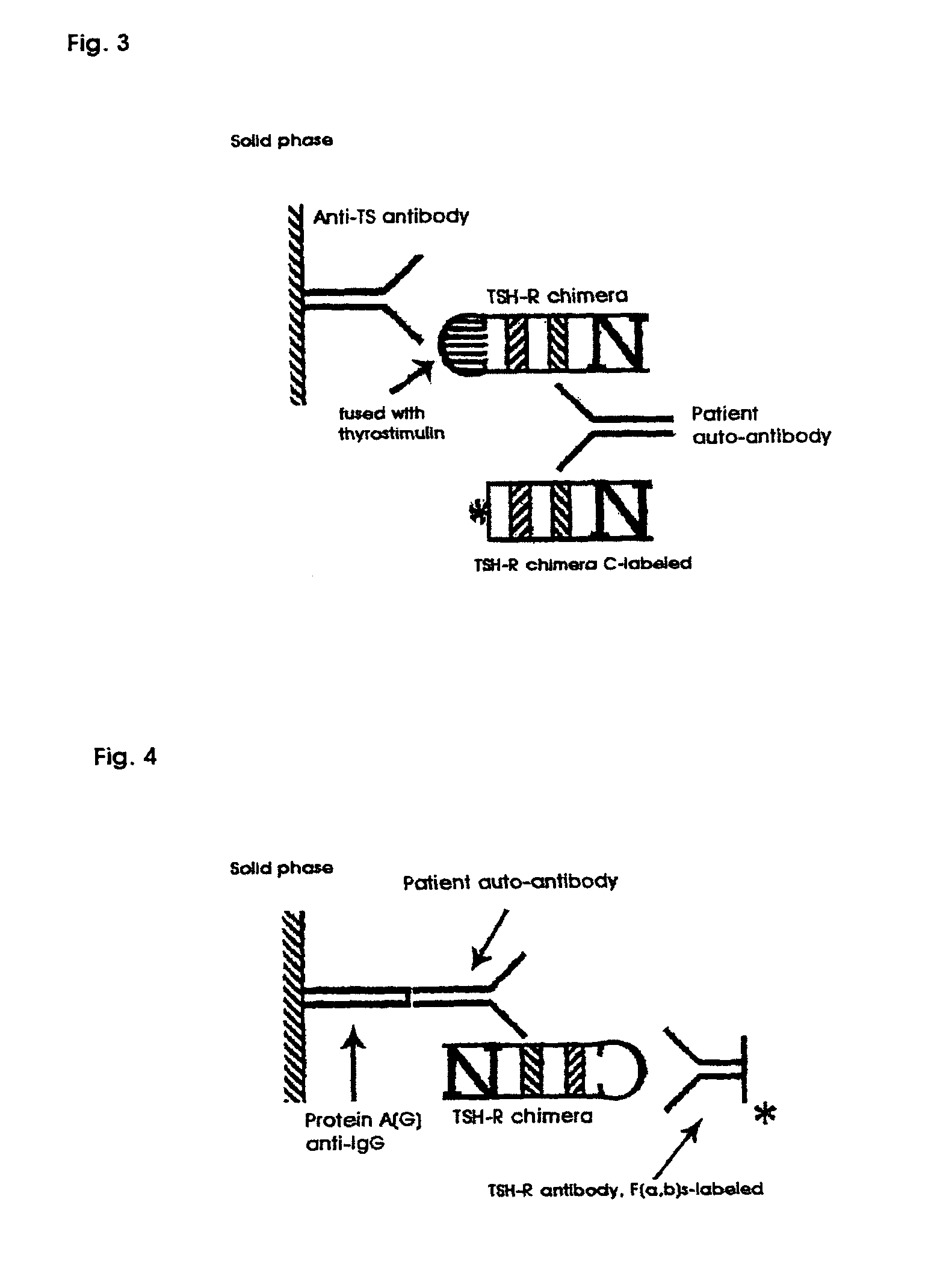
Methods are described for detection of autoimmune antibodies against the TSH receptor using TSH receptor chimeras, which preferably only contain the extracellular portion of the TSH wild type receptor modified as chimera, and are modified by highly immunogenic peptide residues or by enzymes suitable for detection, wherein the determination methods described allow simple detection of stimulating, blocking and neutral autoimmune antibodies.
Owner:LOOS ULRICH
Kit and method for detecting autoimmune antibody of type-I diabetes mellitus
InactiveCN103116030ASimple loading procedureImprove featuresBiological testingAutoantibody productionLuciferase
The invention provides a kit and method for detecting an autoimmune antibody of the type-I diabetes mellitus and belongs to the technical field of biochemical medicine detection. The method comprises the following steps of: adding a luciferase as an antibody fusion protein, wherein the luciferase as the antibody fusion protein is generated by 293 cell culture and can be specifically combined with a diabetes mellitus autoantibody in serum of a patient; then, adding protein-A agarose, depositing a fusion protein as an antibody compound, centrifuging, and then, absorbing the uncombined fusion protein from a supernatant liquid; and then, adding a luciferase substrate, and detecting the fluorescence intensity by using a fluorescence detection instrument to finally measure the content of the diabetes mellitus autoantibody in a sample to be detected. Compared with the traditional HPLC (High Performance Liquid Chromatography), a micro-quantitative fluorescence detection instrument used for detecting in the method has the advantages of simplicity in operation, no need of uncovering to block a pollution way, high sensitivity and signal to noise ratio, stable and reliable measured value and capability of ensuring reliable experiment result and safety of operating personnel and meeting the requirements of micro quantity and regent saving.
Owner:山东东兴生物科技股份有限公司
Autoimmunity antibody detection kit and detection method
The invention relates to an autoimmunity antibody detection kit and a detection method. The detection kit comprises an antigen-coated strip-shaped solid phase carrier, an enzyme-labeled conjugate, an enzyme substrate, a confining liquid and a buffer solution. The detection method comprises the following steps that: a sample to be tested serum or plasma and the antigen-coated strip-shaped solid phase carrier are incubated at room temperature; after incubation, the solid phase carrier is washed with the buffer solution; the enzyme-labeled conjugate and the solid phase carrier are incubated at room temperature; after incubation, the solid phase carrier is washed with the buffer solution; the solid phase carrier and the enzyme substrate are incubated at room temperature; after incubation, the solid phase carrier is washed with deionized water and dried; and the dried solid phase carrier is put into a detection system. The detection method provided by the invention is simple, rapid and sensitive to operate, and the detection kit and the detection method have good specificity and low requirements for operators; and the interpretation of detection results are basically unaffected by the professional knowledge and experience of testers and other factors, therefore, the detection kit and the detection method can be widely applied to all levels of medical inspection places, especially primary-level medical and health care institutions.
Owner:高翔 +1
Anti-Autoimmune Antibodies for Treatment of Pemphigus
ActiveUS20100135948A1Inhibit expressionInhibit bindingAntibacterial agentsOrganic active ingredientsDesmoyokinAutoantibody production
This invention relates to compositions and methods for the use of anti-autoimmune reagents that specifically bind to anti-desmoglein antibodies, which are responsible for both pemphigus vulgaris and pemphigus foliaceus. In addition, the invention relates to methods and compositions for inhibiting the expression or function of a variable region of an anti-desmoglein (anti-Dsg) pathogenic autoantibody.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for detecting autoimmune antibodies against TSH receptors and novel TSH-receptor chimaeras
The invention relates to a method for identifying autoimmune antibodies against TSH receptors with the aid of TSH receptor chimaeras, which, preferably, contain chimaeras which are embodied only in the form of the modified extracellular fraction of a TSH savage-type receptor and are modified by highly immunogenic peptide groups or by detection of suitable enzymes, wherein the inventive determining method makes it possible to easily detecting stimulating, blocking and neutral autoimmune antibodies.
Owner:乌尔里希洛斯
Diagnosis and treatment of autoimmune disease
InactiveUS20130109107A1Reduce the amount requiredCarrier-bound/immobilised peptidesBiological testingAutoimmune thyroid diseaseBinding site
The detection of parietal cell autoimmune antibodies comprising an ATP4A D3.2 subdomain binding site can diagnose autoimmune body gastritis and / or pernicious anemia with extraordinary sensitivity and specificity that is far superior to existing commercial assays. Further, the assay has diagnostic applications for use in diagnosing type 1 diabetes, thyroiditis and Addison's disease. As pernicious anemia is typically a disease of the elderly, detection of parietal cell antibodies may precede clinical disease by many years if not decades, thereby allowing the initiation of therapeutic interventions such as vitamin B12 administration to prevent the development of pernicious anemia or immunologic interventions to prevent type 1 diabetes and its complications.
Owner:UNIV OF COLORADO THE REGENTS OF
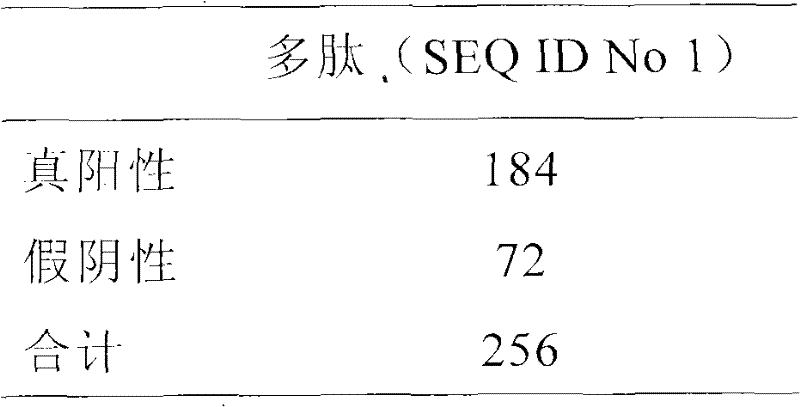
Polypeptide composition for detecting immune antibody of rheumatoid arthritis in vitro
ActiveCN101819201AFacilitate in vitro detectionHigh affinityBiological testingHybrid peptidesArginineSide chain
The invention discloses a polypeptide composition for detecting an immune antibody of rheumatoid arthritis in vitro. At least one arginine side chain in various polypeptide sequences is modified to be electro-neutral or electro-negative amino acid. Cyclic polypeptide is produced by forming a disulfide bond with two non-adjacent cysteine side chain sulfhydryl groups in the sequence. The polypeptide not only can be combined with the autoimmune antibody of the rheumatoid arthritis, but also have high affinity to human leukocyte antigen DR (HLA-DR). Experiments prove that the specificity for the combination of the polypeptide and the autoimmune antibody of the rheumatoid arthritis is more than 90 percent and the flexibility for the detection of the autoimmune antibody of the rheumatoid arthritis is more than 75 percent. Compared with the similar products sold on the market, the polypeptide composition has obviously improved sensitivity so as to more contribute to detecting the immune antibody of the rheumatoid arthritis in vitro.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Kit for detecting autoimmune antibodies at throughput
InactiveCN102053154ASimple production methodFast production methodBiological testingAntigenHigh flux
The invention relates to a kit for detecting autoimmune antibodies at throughput, which mainly comprises a membrane, wherein a plurality of antigens are subjected to dot printing on the membrane in the self-determined order and in a physical adsorption mode of direct contact; and the membrane can adsorb a plurality of required antigens, and can detect a plurality of autoantibodies simultaneously in high throughput with other components of the kit. In addition, the kit has a simple and quick production method, and low production cost.
Owner:深圳市宏信生物技术有限公司
Anti-autoimmune antibodies for treatment of pemphigus
This invention relates to compositions and methods for the use of anti-autoimmune reagents that specifically bind to anti-desmoglein antibodies, which are responsible for both pemphigus vulgaris and pemphigus foliaceus. In addition, the invention relates to methods and compositions for inhibiting the expression or function of a variable region of an anti-desmoglein (anti-Dsg) pathogenic autoantibody.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Composition for externally detecting rheumatoid arthritis antibody and application thereof
ActiveCN101726588AFacilitate in vitro detectionHigh affinityBiological testingAntigenHuman leukocyte antigen DR
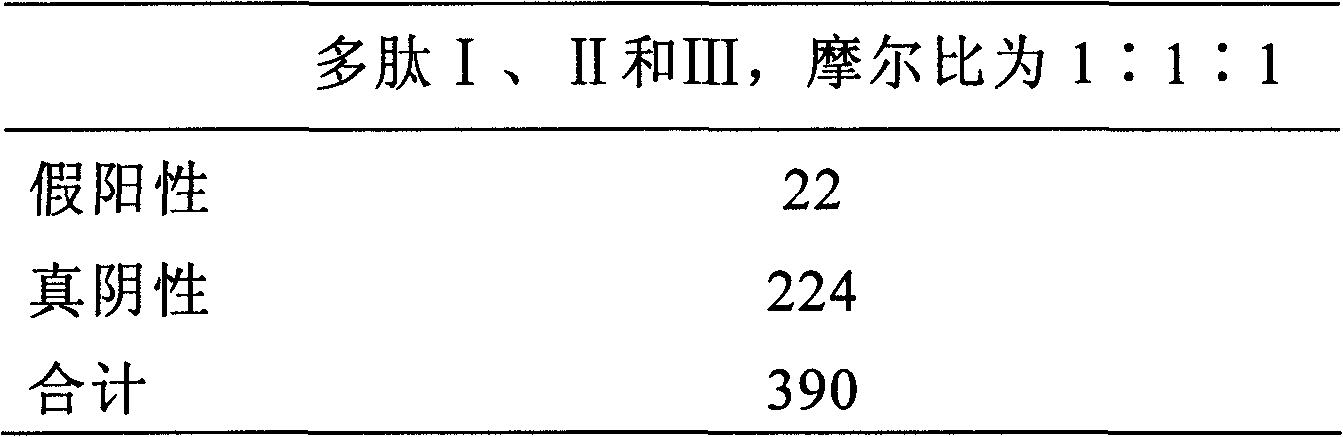
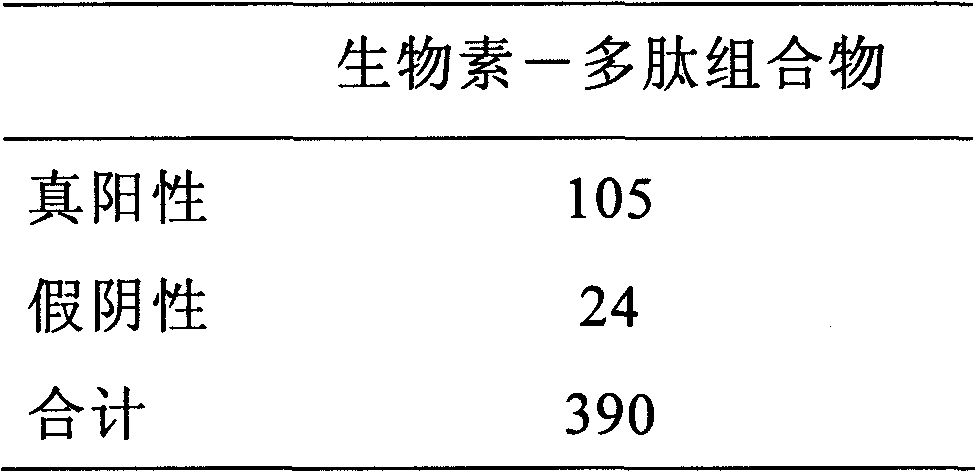
The invention discloses an antigenic composition which comprises a polypeptide I and a polypeptide II, wherein the molar ratio of the polypeptide I to the sum of the polypeptide I and the polypeptide II is 0-1. An annular polypeptide is generated in a mode of forming a disulfide bond from two nonadjacent cysteine side chain hydrosulphonyls on a sequence. The polypeptide not only can be combined with a rheumatoid arthritis autoimmune antibody, but also can simultaneously have high affinity for HLA-DR. Through experimental verification, the specificity of the polypeptide combined with the rheumatoid arthritis autoimmune antibody is larger than 95 percent, and the detection sensitivity for the rheumatoid arthritis autoimmune antibody is larger than 75 percent. Compared with like products sold on the market, the sensitivity of the composition is markedly improved, thereby being more beneficial to externally detecting the rheumatoid arthritis autoimmune antibody.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Autoantibodies utilized as carrier agents for pharmaceutical compounds used in cancer treatment
ActiveUS7799327B2Non-immunogenicRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsImaging agentCytotoxicity
This invention describes a method whereby human autoimmune antibodies are used as carrier compounds to deliver imaging agents and pharmaceutical drugs to the tumor in the human patient. These autoantibodies have the propensity to localize in necrotic areas of tumors but not in healthy normal tissues. By combining various pharmaceutical agents with these carrier proteins it is possible to localize these agents within the necrotic areas of tumors in cancer patients. The carrier proteins may be combined with a variety of imaging agents for detection and diagnosis of tumors, and / or with a variety of radioactive or cytotoxic compounds for cancer treatment.
Owner:SMITH HENRY JOHN +1
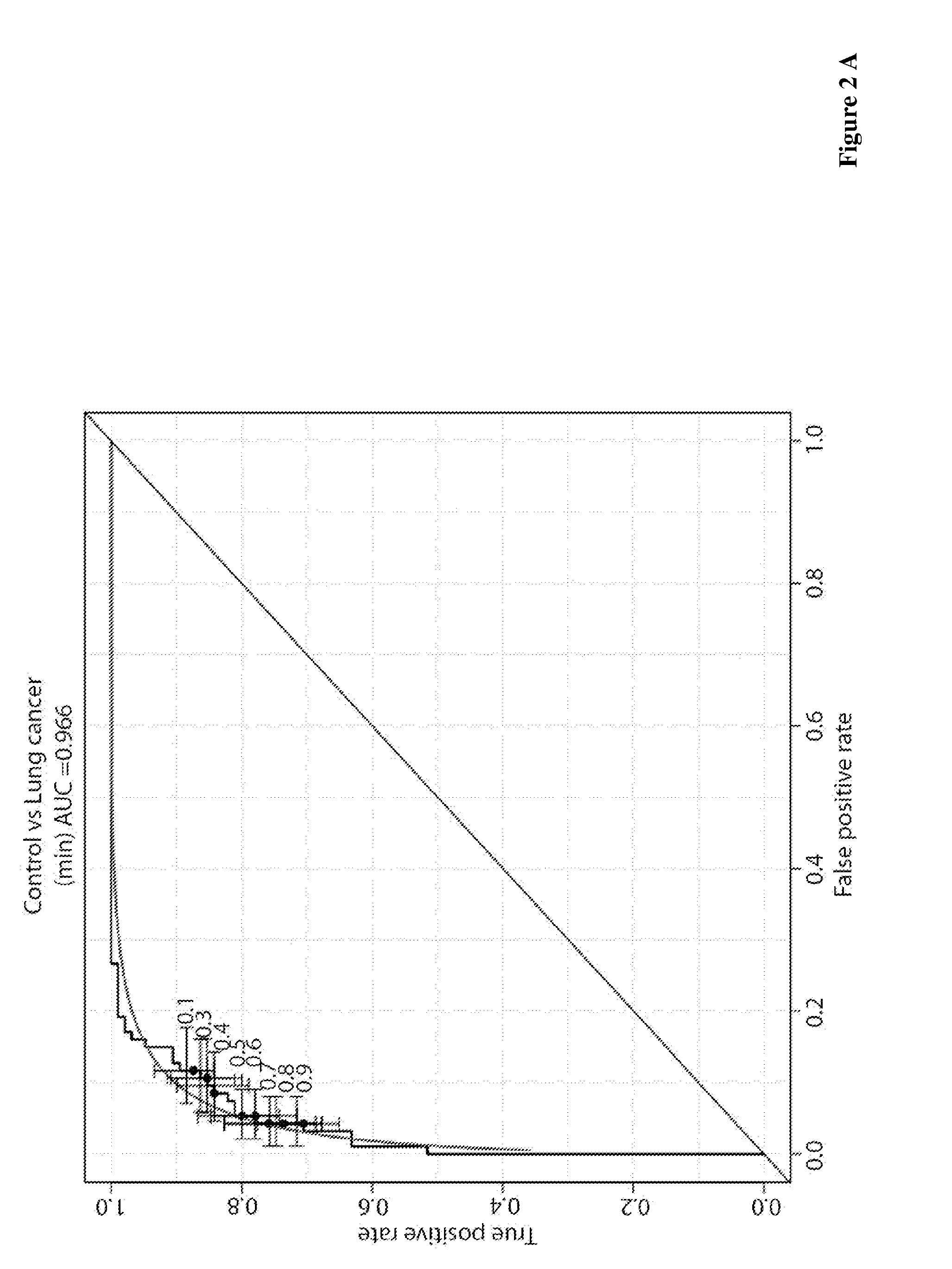
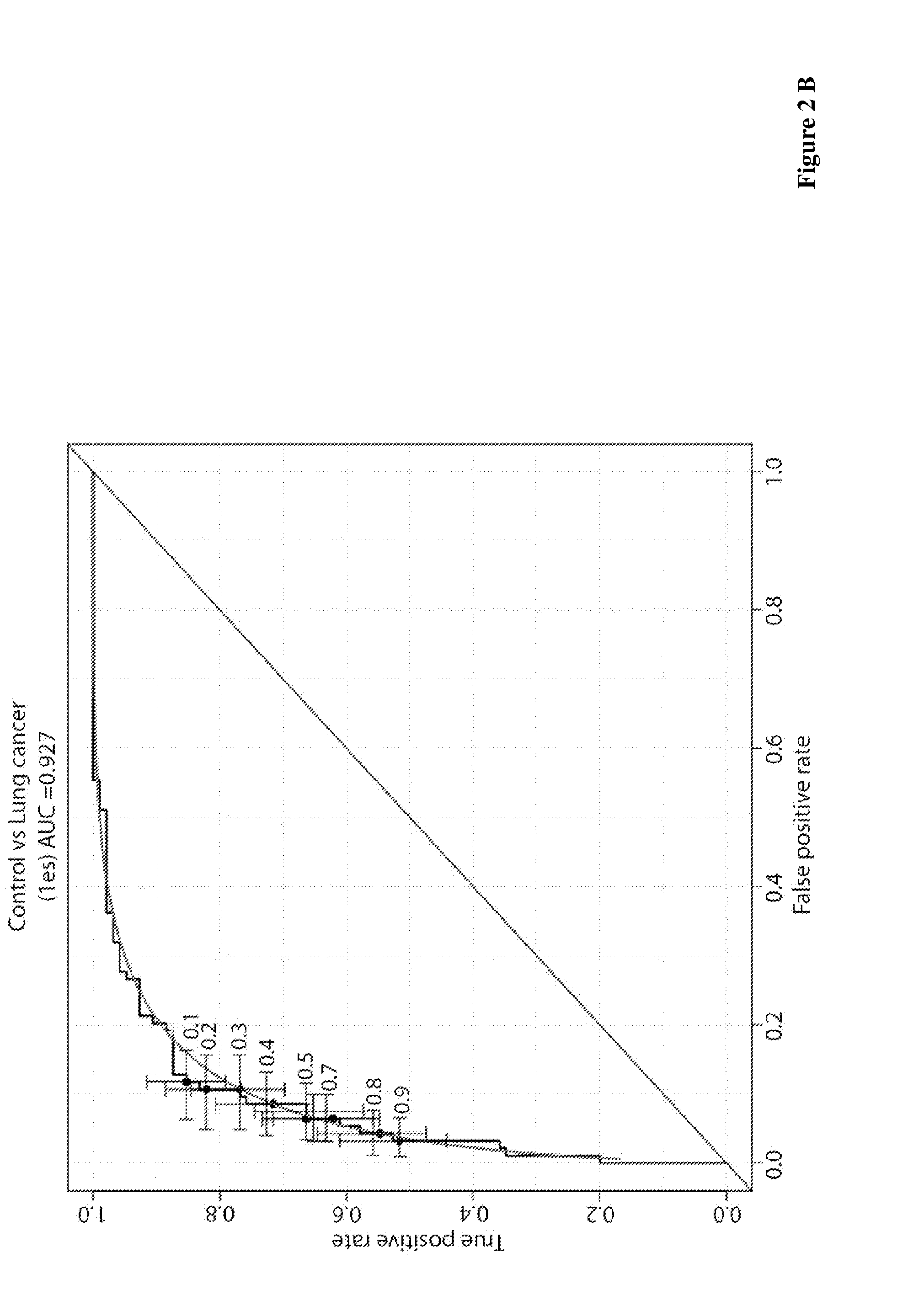
Lung cancer diagnosis
ActiveUS20160282347A1Rapid and reliable and sensitive and specificDisease diagnosisAnimals/human peptidesSerum samplesLarge fragment
The present invention relates to methods for detecting antibodies, methods for diagnosing lung cancer and kits for lung cancer diagnosis. The methods of the invention are based on a blood or serum sample of a subject. According to a preferred embodiment, the invention uses a combination of different peptides comprising an amino acid stretch of BARD1, short peptides and / or larger fragments thereof. In preferred embodiments, the methods of the invention comprise measuring the amount of autoimmune antibodies in the sample binding to each of the different peptides and applying a statistically determined assessment for making the diagnosis.
Owner:BARD1AG
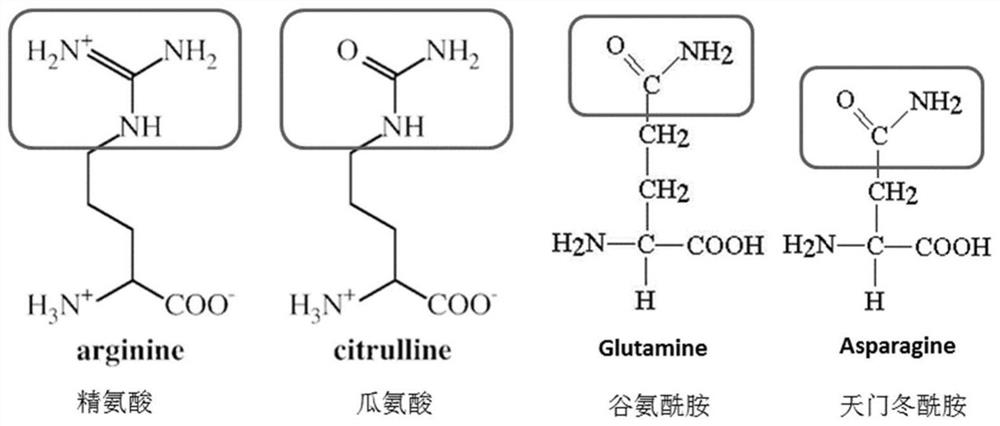
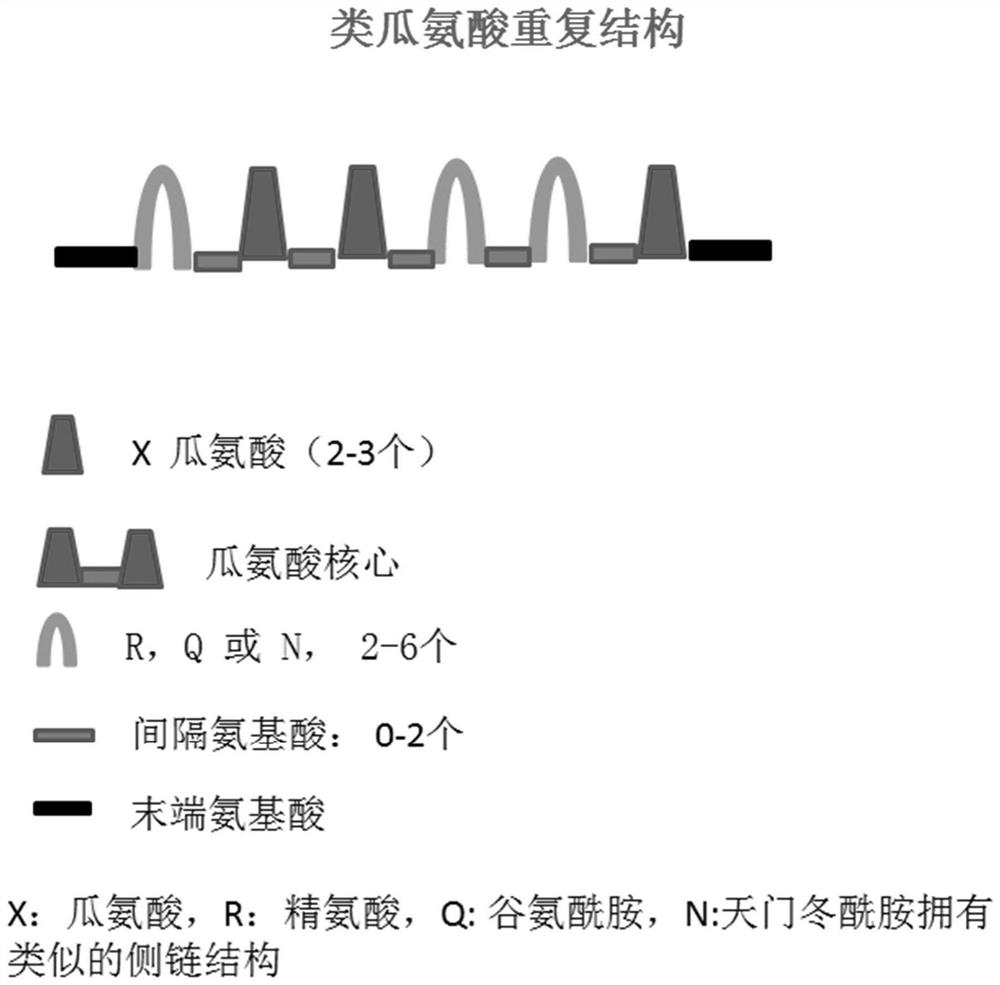
Specific polypeptide related to rheumatoid arthritis and application thereof
ActiveCN111868073AHigh sensitivityImprove featuresPeptide-nucleic acidsDepsipeptidesAntiendomysial antibodiesArthritis
A specific polypeptide related to rheumatoid arthritis and application thereof are provided. The specific polypeptide includes citrulline, wherein at least one side of the citrulline is connected witha citrulline-like amino acid, and a spacer amino acid is also provided between the citrulline and the citrulline-like amino acid. The specific polypeptide provided has high sensitivity and specificity in identifying autoimmune antibodies of patients with rheumatoid arthritis, and can be directly used for the detection of RA autoimmune antibodies, and can also be used to immunize animals to produce polyclonal antibodies or monoclonal antibodies that can be used to detect citrullinated proteins, and to produce identification kits for citrullinated proteins in rheumatoid arthritis serum, joint fluid and body fluids.
Owner:GUANGZHOU LDEBIO TECH CO LTD
Antigen-immobilized matrix membrane, kit comprising the same for detecting antinuclear antibody spectrum related to autoimmune diseases and purpose thereof
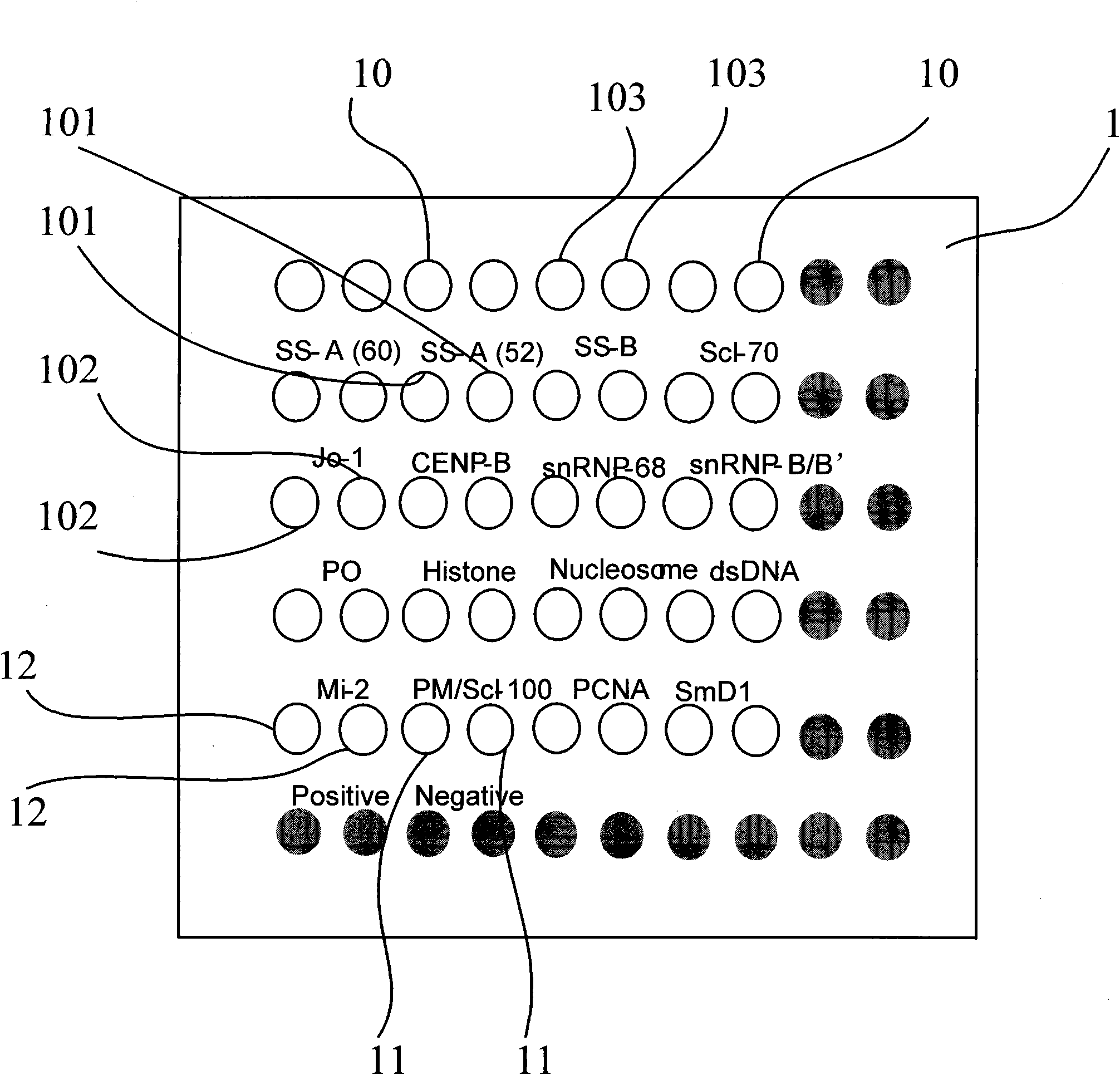
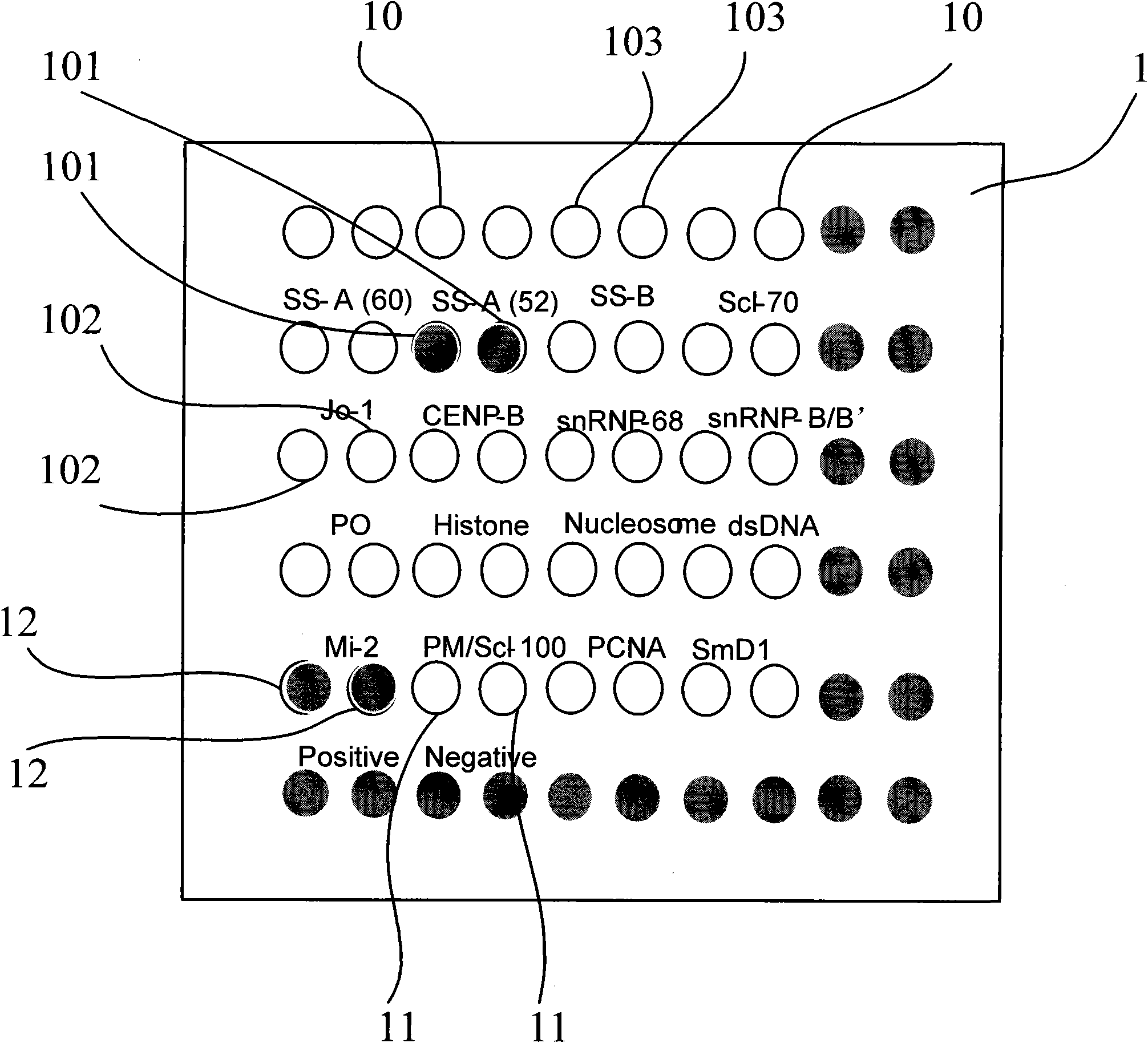
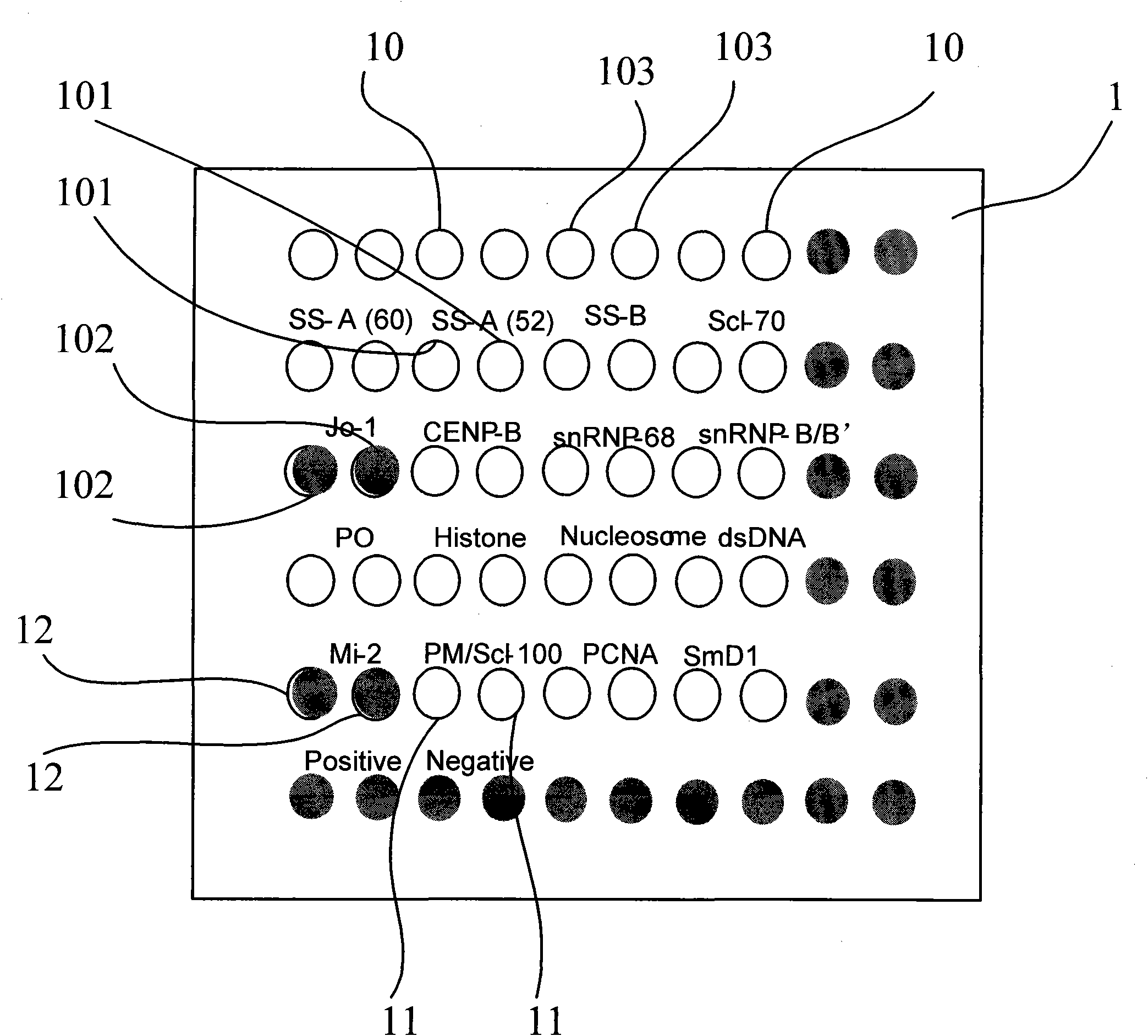
PendingCN110346576AStrong specificityIncreased sensitivityDisease diagnosisBiological testingDiseaseAntigen testing
The invention discloses an antigen-immobilized matrix membrane, a kit comprising the same for detecting an antinuclear antibody spectrum related to autoimmune diseases and purpose thereof. The antigen-immobilized matrix membrane comprises but not only comprises following 22 mutually independent antigen detection lines including double-stranded DNA, histone, ribosome P0 protein, PCNA, SmD1, SmD3, SmB / B', snRNP-A, snRNP-C, snRNP68 / 70, SSA / Ro60, SSA / Ro52, SSB / La, Jo-1, PL-7, PL-12, PmScl, Scl-70, CENP-A, CENP-B, RA33 and PR3. When the kit containing the antigen-immobilized matrix membrane is usedfor detection, 22 kinds of autoimmune antibodies can be detected simultaneously; and thus diagnosis of eight kinds of common diffuse connective tissue diseases is assisted.
Owner:英诺诊断有限公司
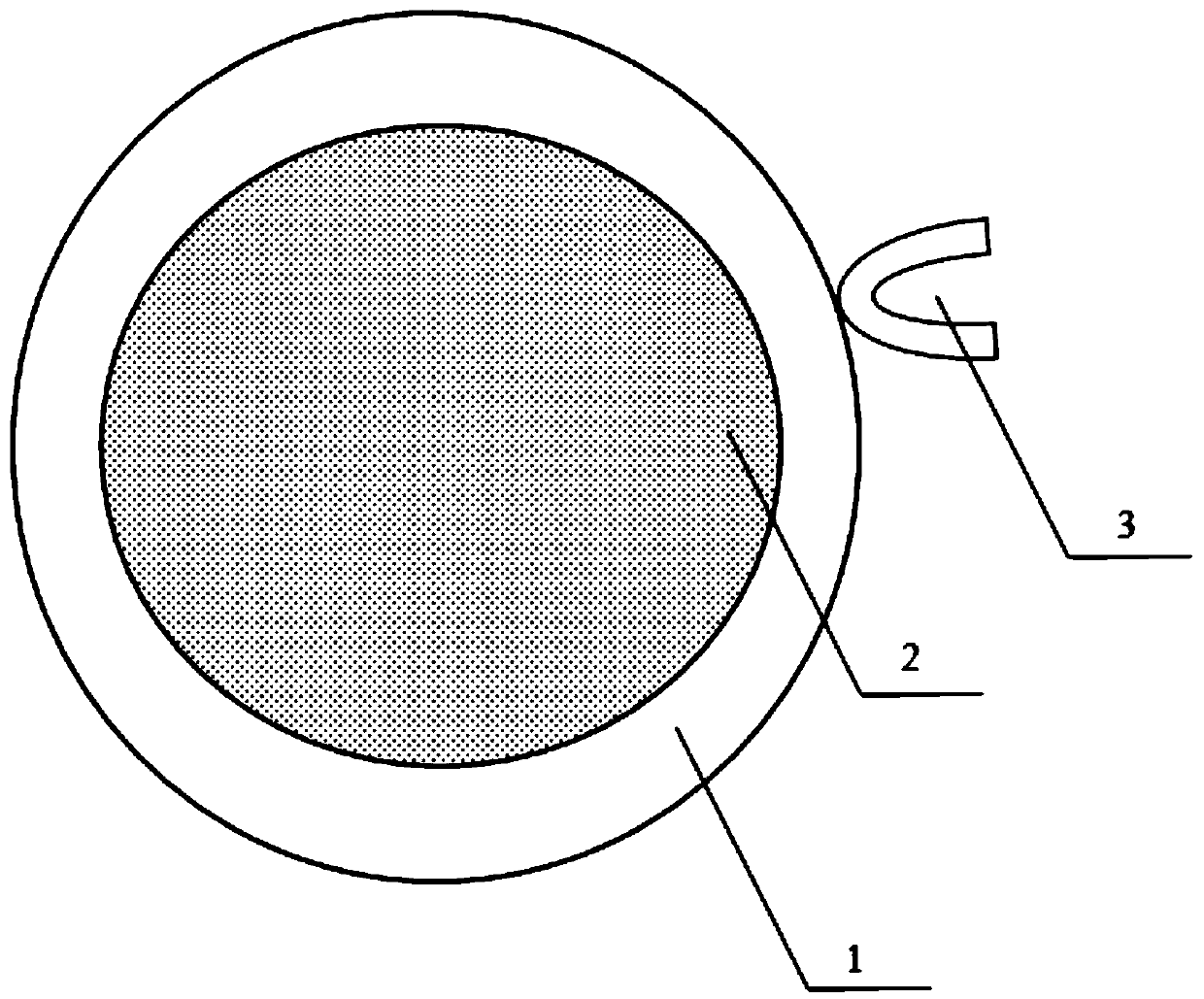
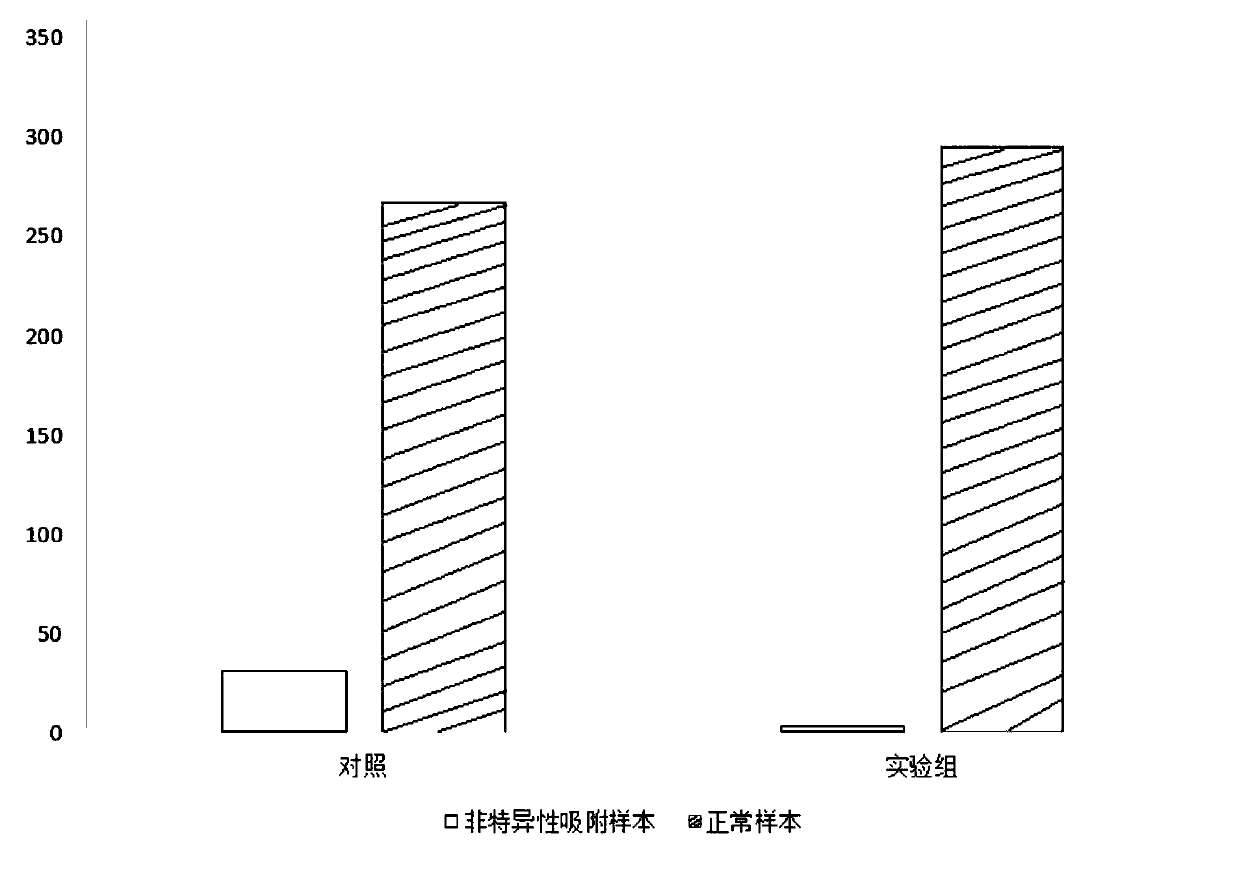
Treatment method for reducing non-specific adsorption in autoimmune antibody detection and kit thereof
PendingCN111521777AReduce false positive rateImprove the detection rateMaterial analysisAntigenSpecific adsorption
The invention belongs to the technical field of immunodetection, and discloses a treatment method for reducing non-specific adsorption in autoimmune antibody detection and a kit thereof. The kit for reducing detection of the autoimmune antibody, provided by the invention, is formed by combining polymer particles, a magnetic particle coupling antigen, AP-labeled anti-human IgG, an autoantibody IgGantibody and a Tris buffer solution after mutual dissolution. According to the detection kit for reducing the autoimmune antibody, polymer particles and magnetic particles in the kit are added to competitively bind non-specific substances to a detection target object; the non-specific adsorption capacity of the magnetic beads on the surfaces of the magnetic particles is further reduced; the falsepositive probability of the detection kit caused by non-specific adsorption in the autoimmune antibody detection process is remarkably reduced, the influence of non-specific substances on a clinical sample test result is weakened, and the detection rate of the detection kit and the effectiveness and stability of the detection result are improved.
Owner:四川携光生物技术有限公司
Breast cancer autoimmune antibody detection kit and preparation method and application thereof
InactiveCN109752547AAvoid harmMeet needsBiological testingHybrid peptidesGeneral practionerTrue positive rate
The invention relates to a kit. The kit adopts a gene recombinant Renilla and multi-antigen fusion protein to detect an autoimmune antibody in serum, which is related to the breast cancer, has high sensitivity and specificity, provides basis for diagnosis of the breast cancer, and provides scanning conditions for further pathological diagnosis. The kit is rapid and convenient to detect, accurate in detection result and harmless for a patient, has no radio-immunity pollution, can meet requirements of clinical diagnosis and particularly requirements of primary health care institutions and community general practitioners, and has wide market prospect.
Owner:杭州京北生物科技有限公司
Polypeptide combined with immune antibody and application thereof
ActiveCN101812119AHigh detection sensitivityFacilitate in vitro detectionPeptidesMaterial analysisArginineSide chain
The invention relates to a polypeptide combined with an immune antibody. Three arginine side chains in sequences of the polypeptide are modified to be electrically neutral or electronegative amino acid; and two non-adjacent cysteine side chain mercapto groups on the polypeptide sequences forms annular disulfide bonds. The polypeptide can be combined with the autoimmune antibody of rheumatoid arthritis and can have high affinity to HLA-DR. The experimental result shows that the specificity of the polypeptide combined with the autoimmune antibody of the rheumatoid arthritis is over 75 percent. Compared with the like products sold in the market, the polypeptide has the advantage that: the sensitivity of the polypeptide is obviously improved so as to more contribute to the detection in vitro for the autoimmune antibody of the rheumatoid arthritis.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Innovative TSH-R-Ab-kit
ActiveUS8999727B2Improve accuracyImprove expressivenessReceptors for hormonesBiological testingWild typeImmunogenic peptide
Methods are described for detection of autoimmune antibodies against the TSH receptor using TSH receptor chimeras, which preferably only contain the extracellular portion of the TSH wild type receptor modified as chimera, and are modified by highly immunogenic peptide residues or by enzymes suitable for detection, wherein the determination methods described allow simple detection of stimulating, blocking and neutral autoimmune antibodies.
Owner:LOOS ULRICH
Detection method of human myelin oligodendrocyte glycoprotein autoimmune antibody
ActiveCN107271680BImprove detection efficiencyStrong specificityDisease diagnosisBiological testingChaperoninMyelin sheath
The invention discloses a detection method of a self immune antibody. The method comprises the following steps of performing cotransfection cells by expression vectors of allergen protein of an antibody to be detected and a chaperone protein expression vector; after the transfected cells are fixed and after washing, adding serum to be tested for incubation; adding second antibodies for incubation; then, performing analysis, wherein the antigen protein of the antibody to be detected is MOG protein. The detection method has the characteristics of high detection efficiency, high specificity and high sensitivity. The common problem of false positive or false negative detection result in the immunoblotting and enzyme linked immunosorbent assay are successfully solved; very important study and practical values are realized.
Owner:广州敏特生物技术有限公司
Digital microfluidic autoimmune antibody multiple detection system
InactiveCN113181981ALow costImprove reliabilityLaboratory glasswaresBiological testingAntigenBiology
The invention provides a digital microfluidic autoimmune antibody detection system. According to the method, the whole-process autoimmune antibody detection of a sample is carried out on a single chip, all operations related in an experiment process, including sample quantitative distribution, reagent quantitative distribution, solution transfer, incubation reaction, antigen cleaning and the like, are automatically completed by a digital micro-fluidic chip, and finally, a detection result is judged through fluorescence detection. A user only needs to load a sample to the chip, and the whole-process multiple autoimmune antibody detection can be realized.
Owner:JIANGSU LOGILET BIOTECH CO LTD
Polypeptide combined with immune antibody and its application
ActiveCN101812119BFacilitate in vitro detectionHigh affinityPeptidesMaterial analysisArthritis- rheumatoid arthritisSide chain
A polypeptide that binds to immune antibodies, with three arginine side chains modified to be electrically neutral or electronegative amino acids in its sequence. Two non-adjacent cysteine side chain sulfhydryl groups on these polypeptide sequences form a disulfide bond to form a ring. This kind of polypeptide can not only combine with rheumatoid arthritis autoimmune antibody, but also have high affinity to HLA-DR. It is verified by experiments that the specificity of combining the polypeptide with the autoimmune antibody of rheumatoid arthritis is greater than 95%, and the detection sensitivity of the autoimmune antibody of rheumatoid arthritis is greater than 75%. Compared with similar products sold in the market, its sensitivity is significantly improved, which is more conducive to the in vitro detection of rheumatoid arthritis autoimmune antibodies.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Treatment method for reducing nonspecific adsorption of magnetic beads in autoimmune antibody detection
PendingCN113945711AReduce non-specific adsorptionReduce false positive resultsDisease diagnosisBiological testingMagnetic beadSerum binding
Owner:BEIJING H&J NOVOMED
A kind of pharmaceutical composition for treating multiple sclerosis
InactiveCN103977401BReduce the numberReduce activationNervous disorderMetabolism disorderTnf familyBiological activation
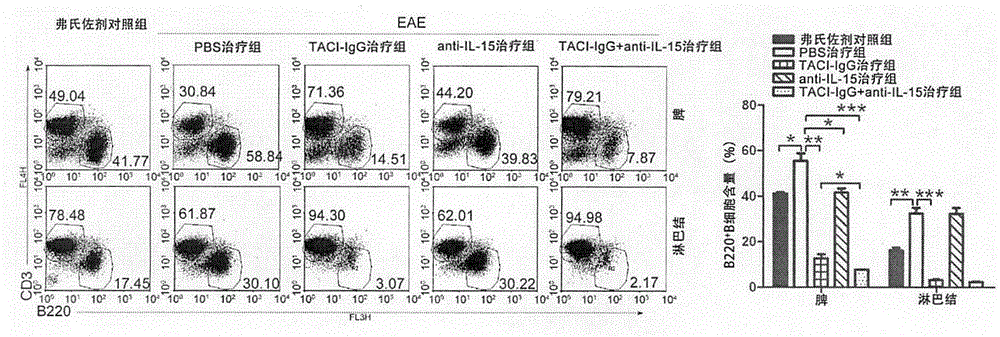
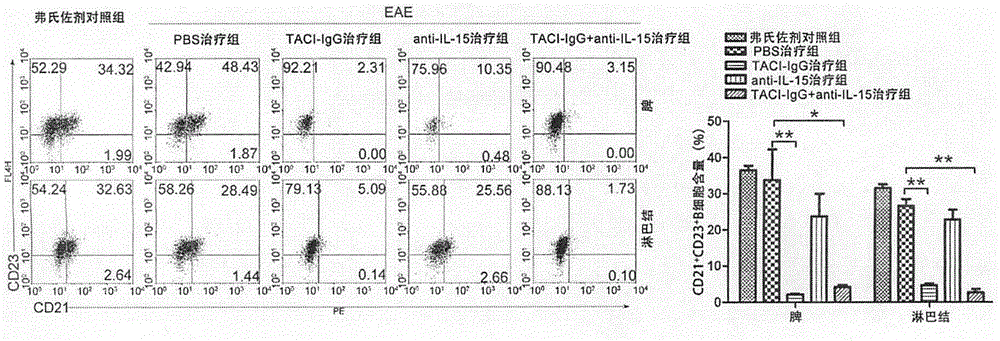
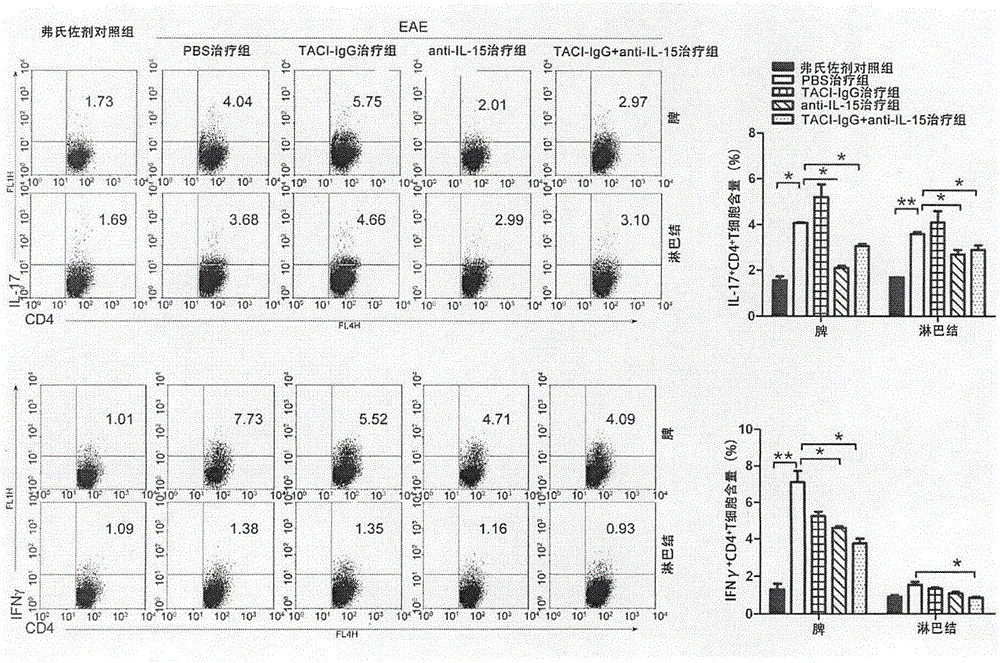
The invention discloses a medicinal composition for treating multiple sclerosis, and the medicinal composition comprises BAFF (B cell activating factor belonging to TNF family) inhibitor (TACI-IgG) and IL-15 inhibitor (anti IL-15 antibody). The medicinal composition is used for treating a murine model EAE (experimental allergic encephalomyelitis) mice subjected to the multiple sclerosis, and the results showed that the medicinal composition can significantly reduce the number of B cells, helper T cells and memory B / T cells in spleen and lymph nodes, inhibits the production of activation and autoimmune antibodies of the B cells, and relieves clinical autoimmune symptoms. The medicinal composition is effective in the treatment of the multiple sclerosis.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Synthetic peptides, methods and kits for diagnosing autoimmune diseases
ActiveUS8557603B2Easy to detectEasy diagnosisAnalysis using chemical indicatorsApolipeptidesAutoimmune conditionAutoimmune disease
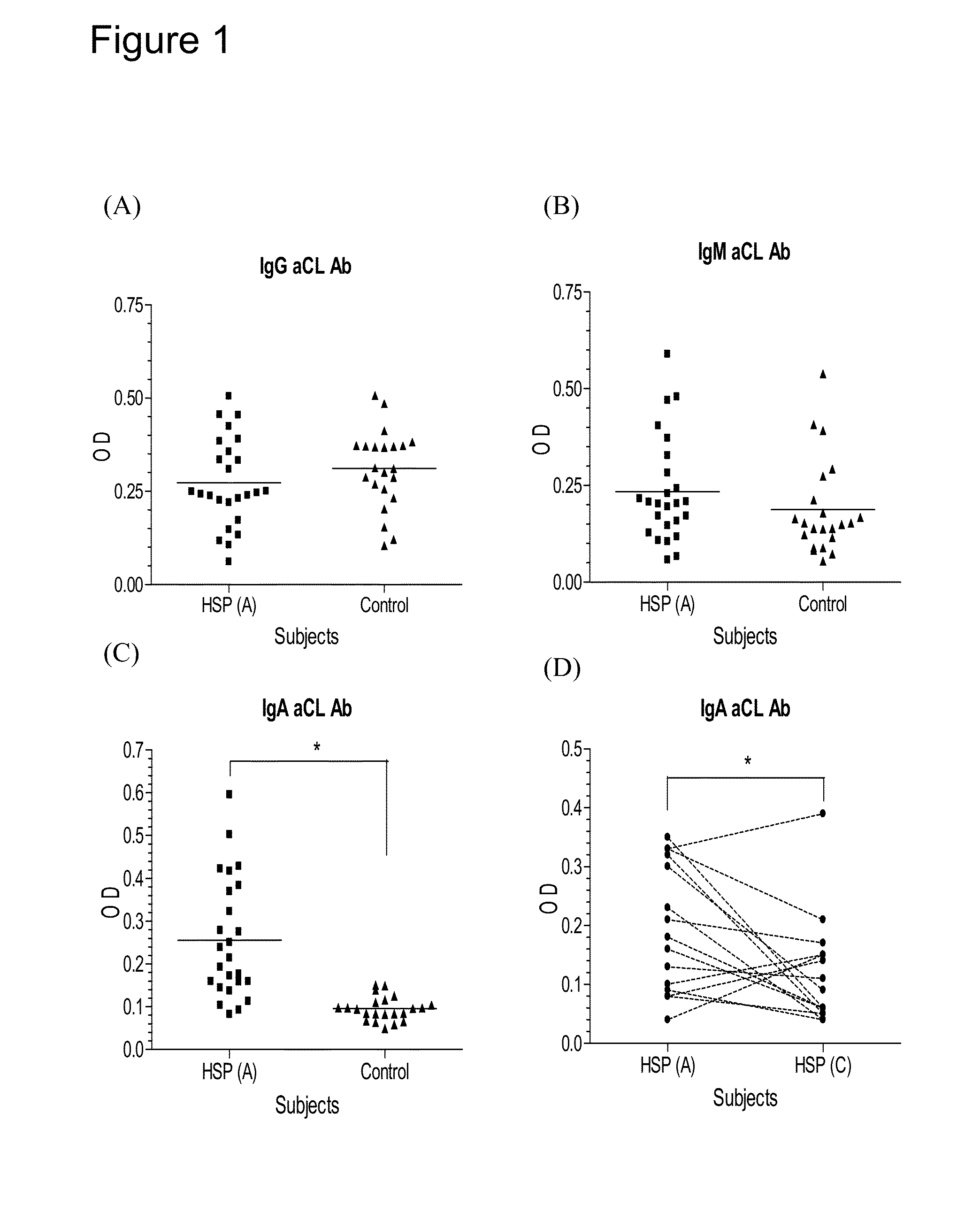
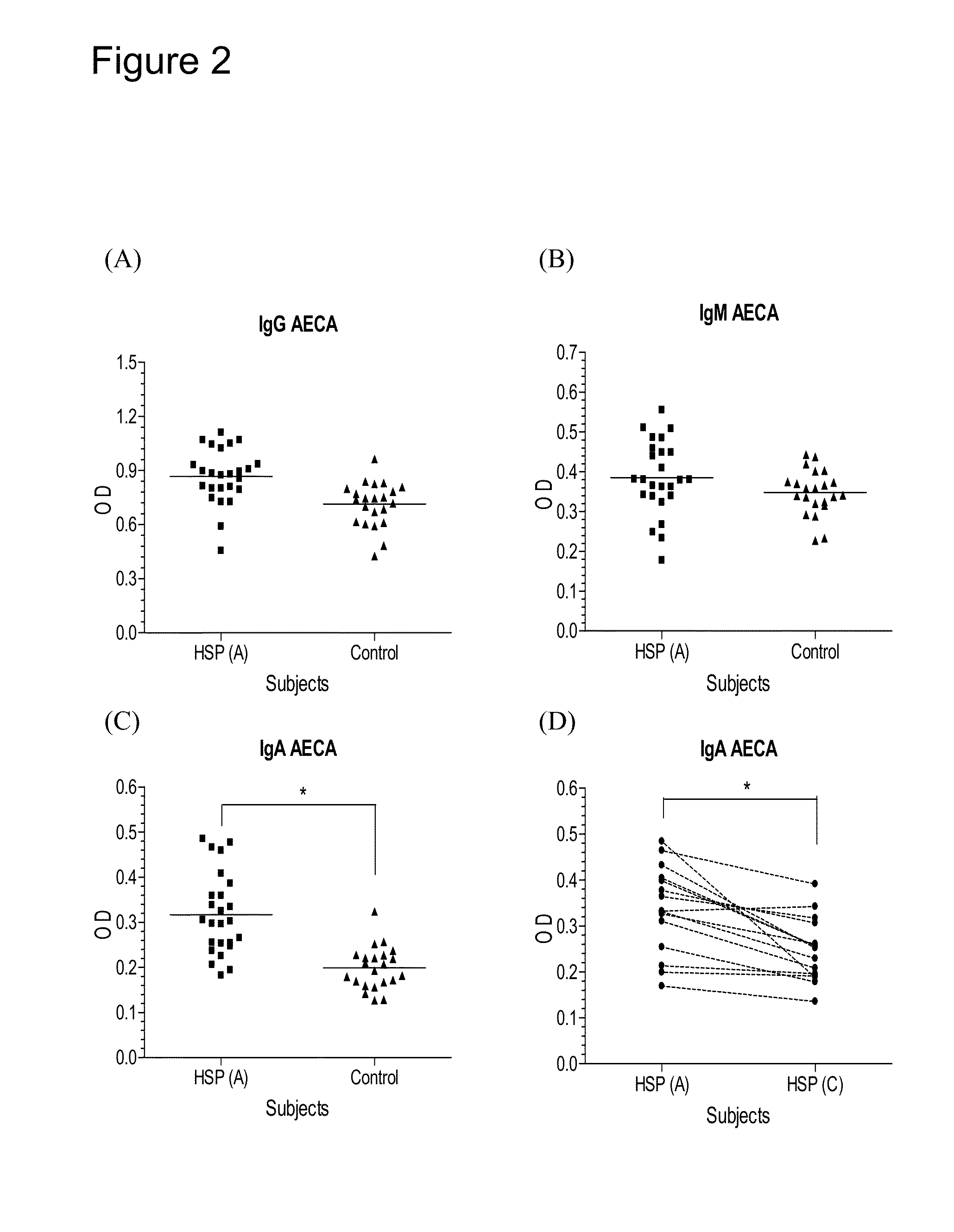
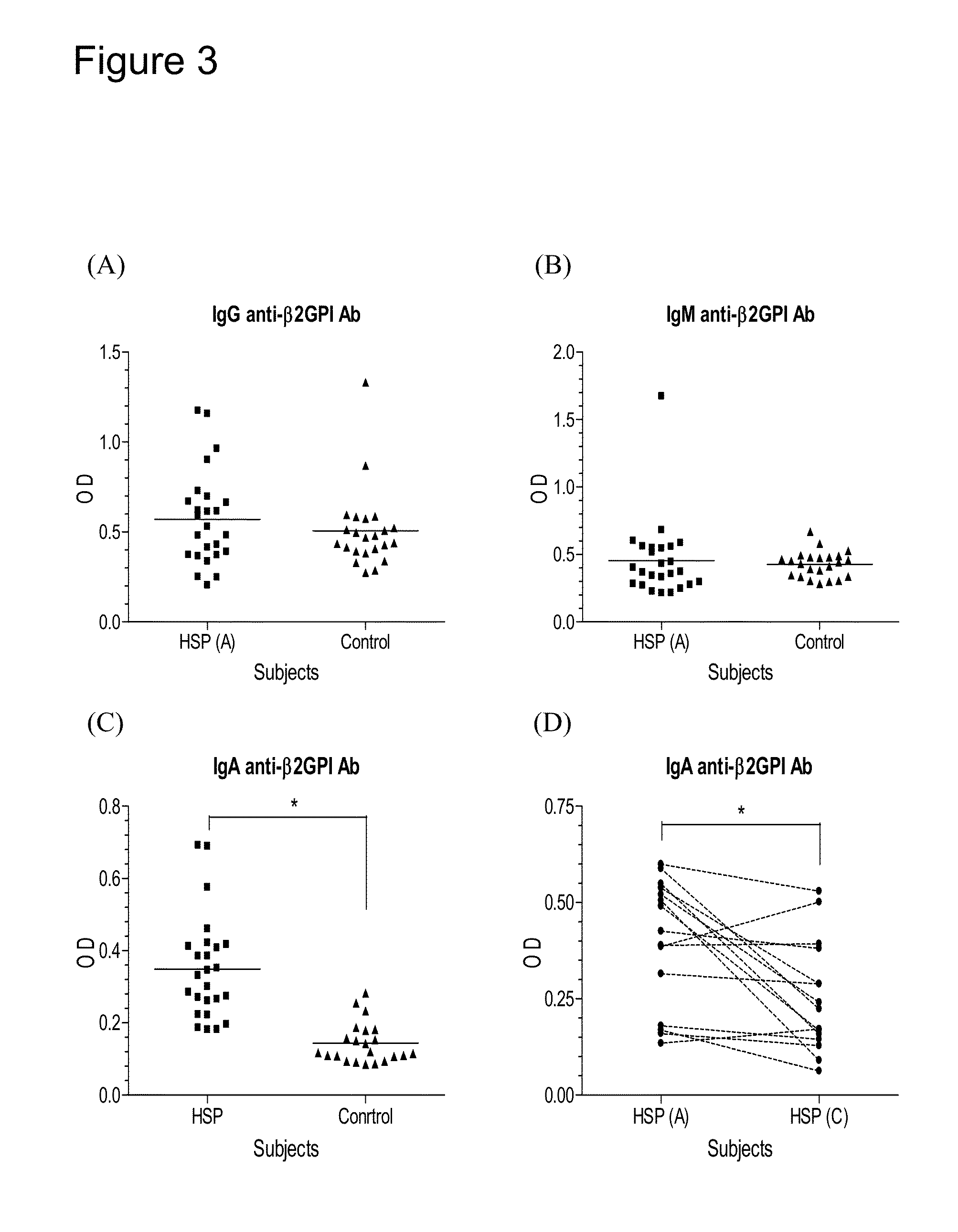
Provided herein are synthetic peptides, methods and kits for easy detecting or diagnosing an autoimmune disease, particularly, Henoch-Schönlein purpura (HSP), based on the detection of autoimmune antibodies with peptides derived from β-2-glycoprotein-1 (β2-GPI).
Owner:FLYSUN DEV
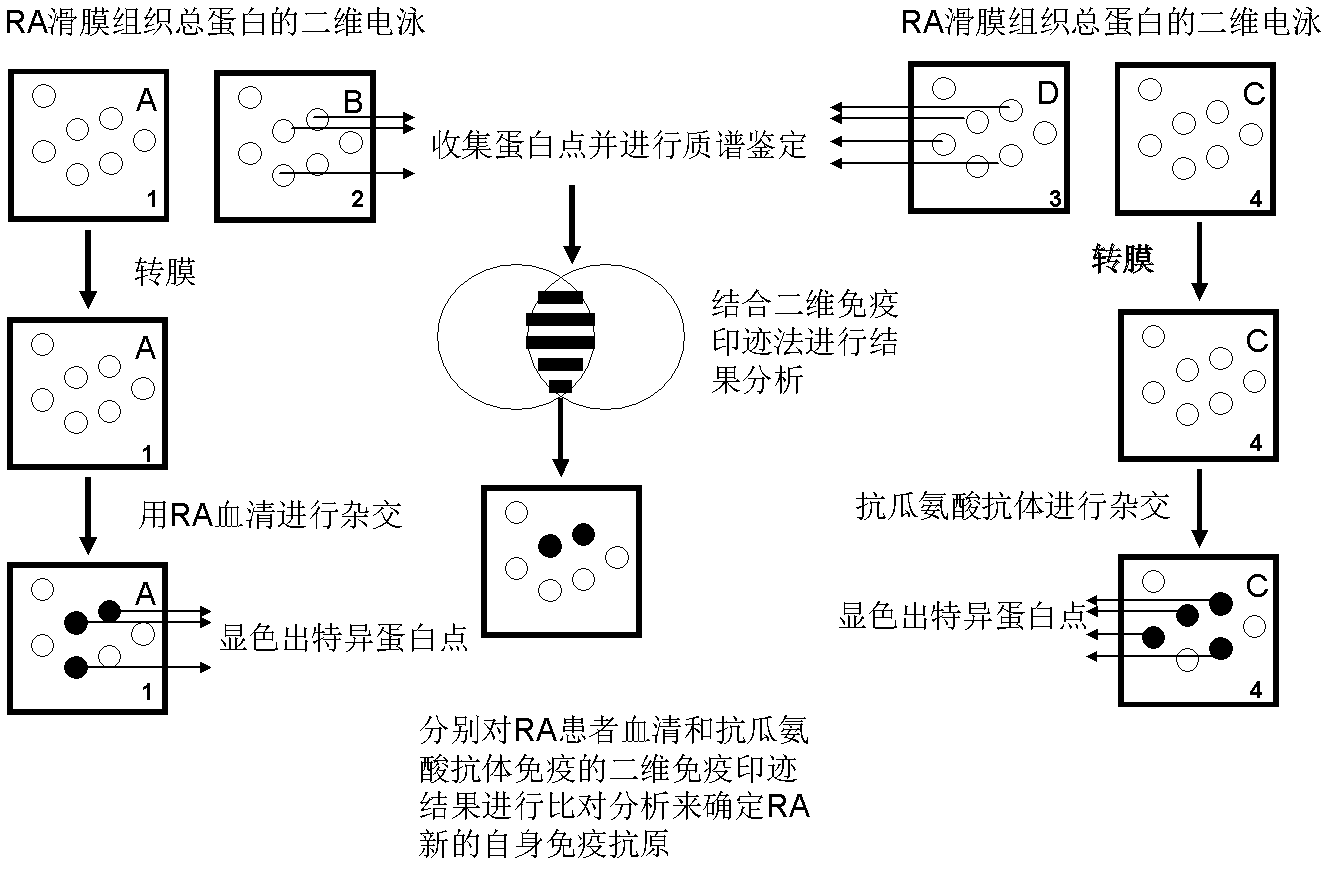
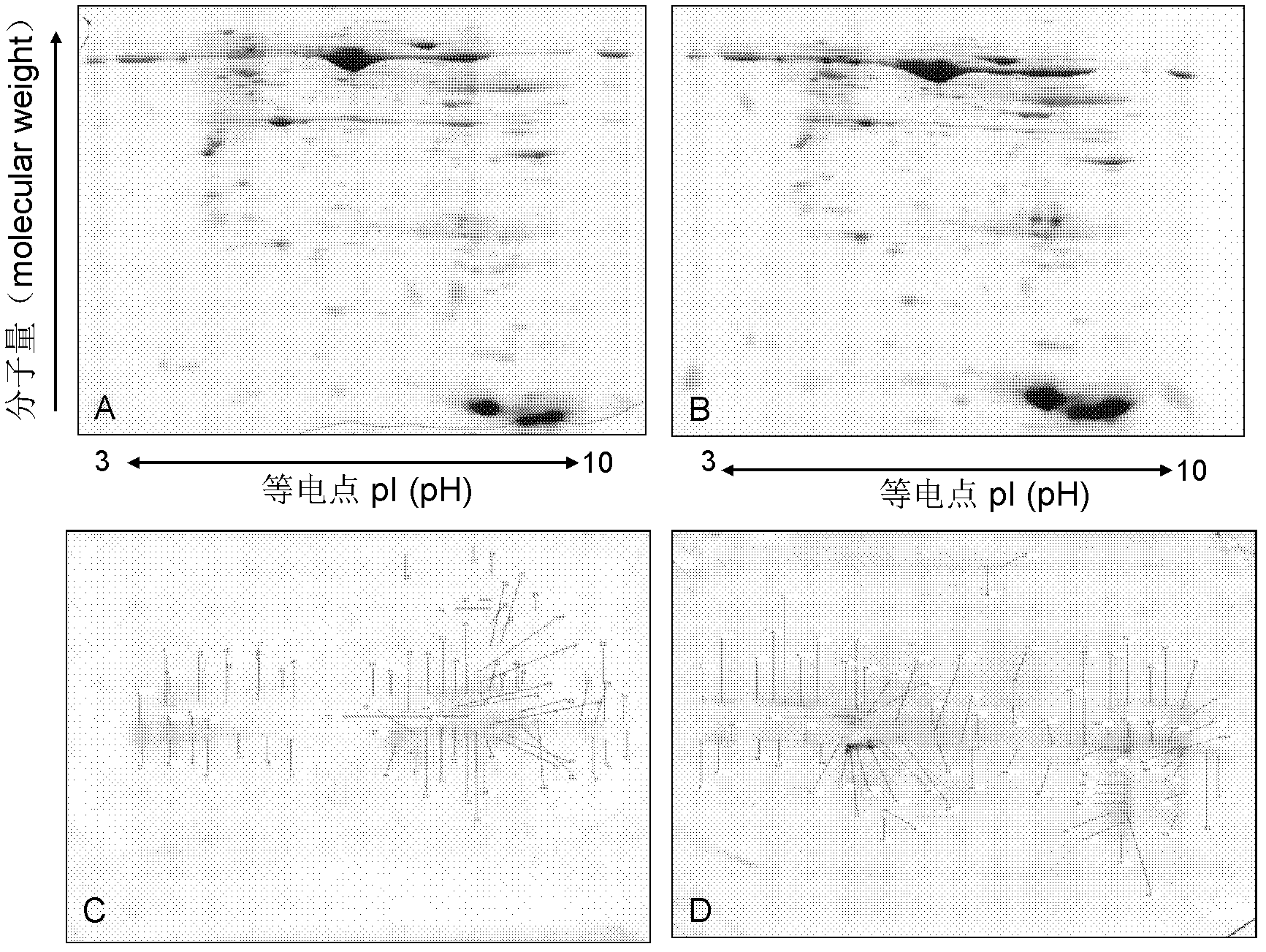
Application of KRT84 self-immune antibody as diagnosis marker for diagnosing and detecting rheumatoid arthritis
The invention discloses application of a KRT84 self-immune antibody as a diagnosis marker for diagnosing and detecting rheumatoid arthritis (RA). According to the application disclosed by the invention, the reality that the expression level of the KRT84-resistant self-immune antibody in the blood of an RA patient is remarkably improved compared with that of a normal person through experimental research (a two-dimensional western blotting method, a proteomic technology and an ELISA (Enzyme-Linked Immuno Sorbent Assay) technology), so that the KRT84-resistant self-immune antibody can be used as the diagnosis marker of RA. When the KRT84 self-immune antibody is applied, immunochemical methods combining an ELISA technology, a colloidal gold test paper and general antigen and antibody specificity can be respectively used for detecting the KRT84 self-immune antibody.
Owner:常晓天 +1
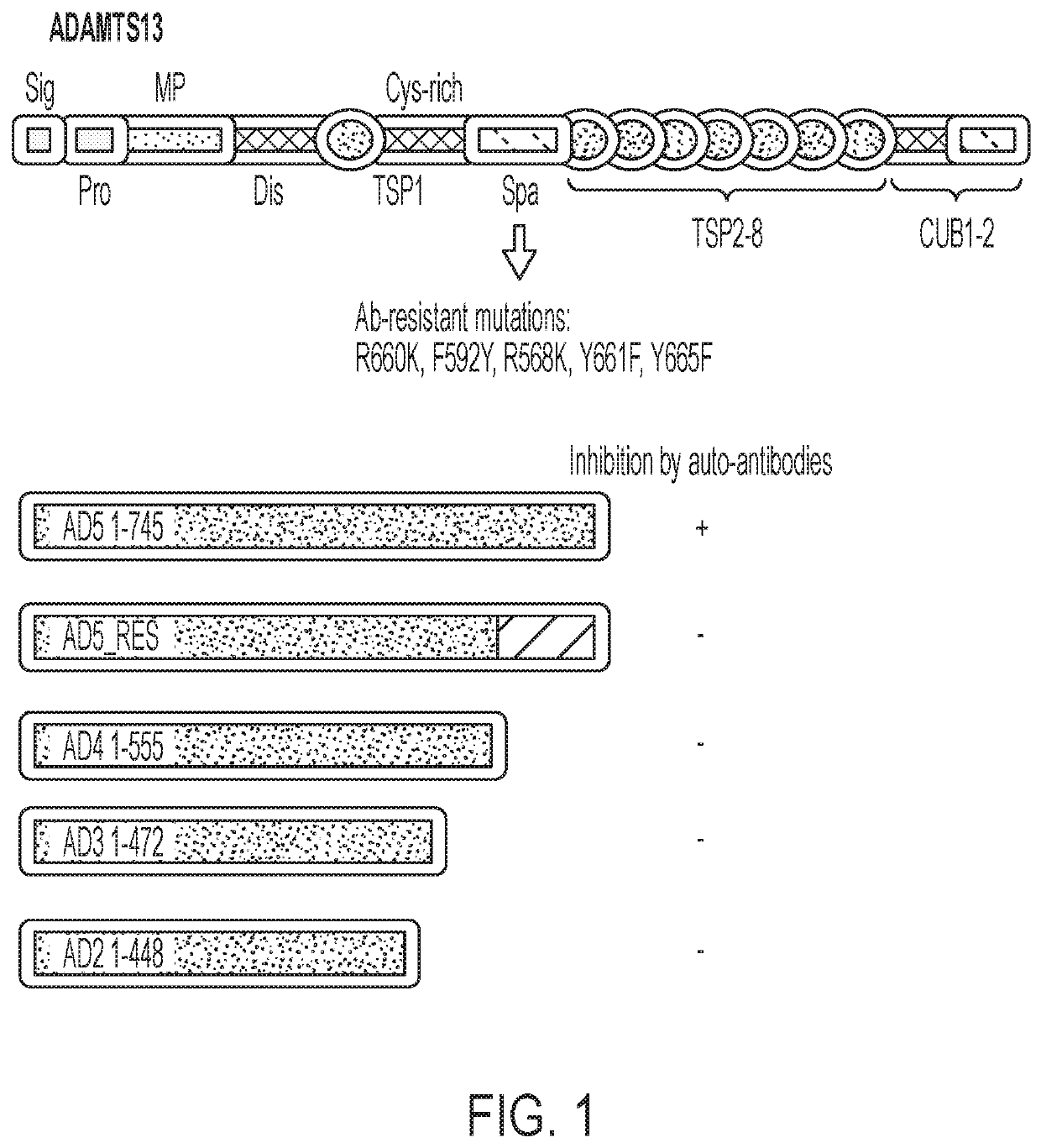
Red blood cells expressing von willebrand factor protease and methods of use thereof
PendingUS20220213462A1Immunoglobulins against blood coagulation factorsFactor VIIFactor VIII vWFRed blood cell
This disclosure provides methods and compositions for treating TTP based on transfusion of a relatively small number of genetically modified red blood cells. The genetically modified red blood cells express a fusion protein including a fragment of ADAMTS13 that is enzymatically active against von Willebrand factor (VWF). The fragments of ADAMTS13 can be resistant to the inhibitors, e.g., the auto-immune antibodies, which are responsible for the acquired form of TTP.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Composite chip for detecting autoimmune antibody of diabetes mellitus, and preparation and detection method thereof
InactiveCN1176377CHas clinical practical valueReduce testing costsBiological testingFluorescence/phosphorescenceDiabetes mellitusFluorescence
Glass thin slice with the multiple reaction ponds is set up on the outer casing of the compound chip for detecting oneself immunity antibody of diabetes. the detecting micro array is built in each pond. Four different kinds of protein solid phase detecting pieces in mini type with sample specification size constitute each detecting micro array. These detecting pieces are the piece of the islet cells, the piece of GAD protein, the PTP protein and the Insulin protein. First, these detecting pieces are prepared, then these pieces are assembled. The detecting method includes two steps: first, the indirect fluorescence method is used; then, the biochip laser co-focusing scanner determines the result. Comparing with the prior art the invention possesses the advantages of the accurate clinical analysis and the low cost.
Owner:XIAN BESTEDIT BIOMEDICAL SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com