Antigen-immobilized matrix membrane, kit comprising the same for detecting antinuclear antibody spectrum related to autoimmune diseases and purpose thereof
An autoimmune disease and antigen detection technology, applied in the field of biomedicine, can solve the problems of incomplete disease types, complex operations, low specificity and sensitivity, etc., and achieve intuitive and accurate result interpretation, high detection efficiency and high specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 kit
[0045] 1. Antigen preparation
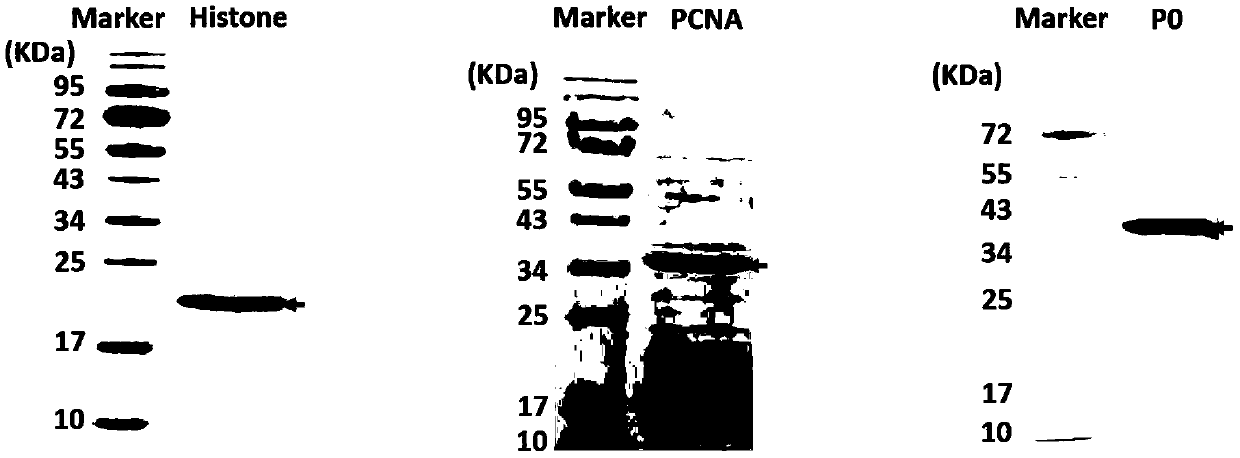
[0046] All autoantigen proteins were expressed in recombinant protein using mammalian cell expression system.
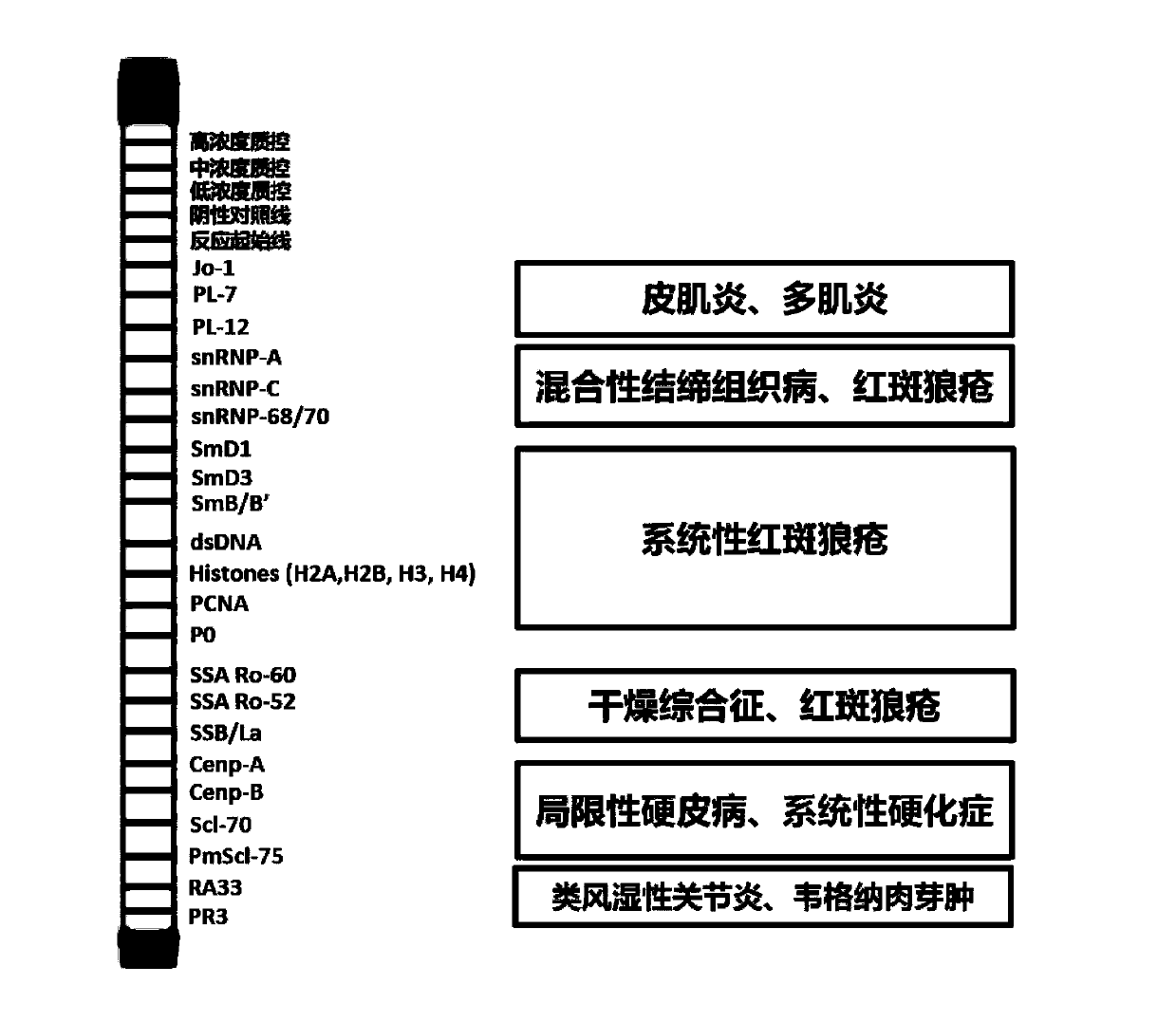
[0047] Entrust the biological company to carry out the sequence of different antigens (Jo-1: Gene ID 3035; PL-7: Gene ID 6897; PL-12: Gene ID 16; SnRNP-A: Gene ID 6627; SnRNP-C: Gene ID 6631; SnRNP68 / 70: Gene ID6625; SmD1: Gene ID 6632; SmD3: Gene ID 6634; SmB / B': Gene ID 6628; dsDNA: PMID: 6272877; H2A: Gene ID 92815; H2B: Gene ID 8349; H3: Gene ID 8350; H4: Gene ID 8359; PCNA: Gene ID 5111; P0: Gene ID 6175; SSA / Ro60: Gene ID 6738; SSA / Ro52: Gene ID 6737; SSB / La: Gene ID 6741; CENP-A: Gene ID 1058; CENP -B: Gene ID 1059; Scl-70: Gene ID 7150; PmScl-75: Gene ID 5393; RA33: Gene ID 3181; PR3: Gene ID 5657) were synthesized and the resulting sequence was cloned into the pcDNA3.1 (+) expression vector by Ampicillin (final concentration 0.1mg / ml) was used to screen the resistant positive cl...
Embodiment 2
[0066] The usage method of embodiment 2 kit
[0067] Sample preparation: human serum or anticoagulated plasma. The samples are stored at 2-4°C. If the test cannot be performed within 24 hours, the samples should be stored at -20 or -80°C. Specimens with severe hemolysis, floc or mold will affect the test results and should not be used.
[0068] Washing solution preparation: dilute 1 part of 20× concentrated washing solution with 19 parts of distilled water, mix well to prepare a standard washing solution (need to be prepared and used immediately).
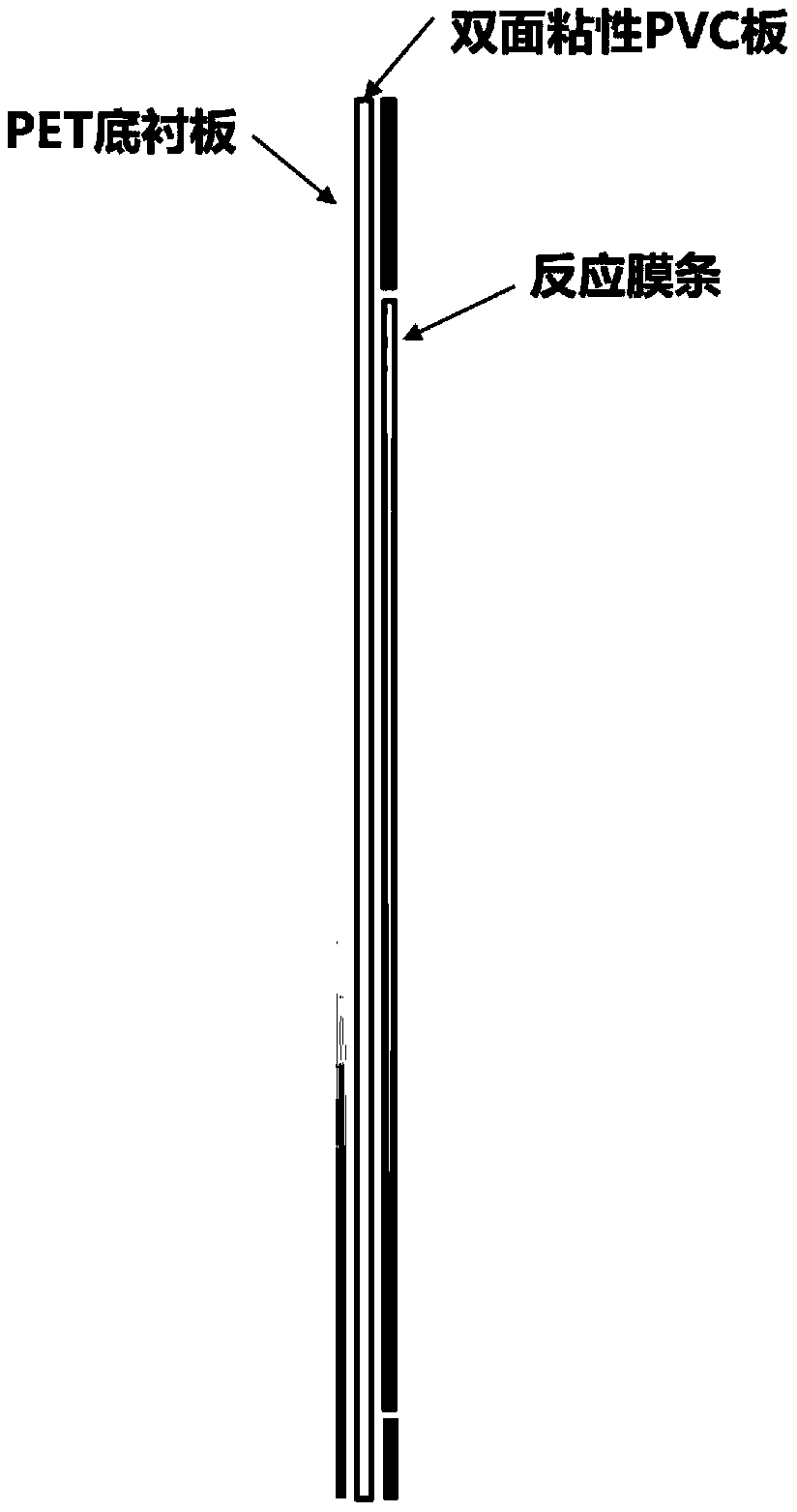
[0069] (1) Put the reaction membrane strip into a semi-closed independent reaction groove in the reaction incubation plate, add 1ml of standard washing solution, shake gently until the membrane strip is completely wet, then discard the dilution solution.
[0070] (2) Dilute the sample to be tested with the sample diluent at a ratio of 1 (sample serum): 250 (diluent), and add 1ml of the diluted sample to the membrane strip reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com