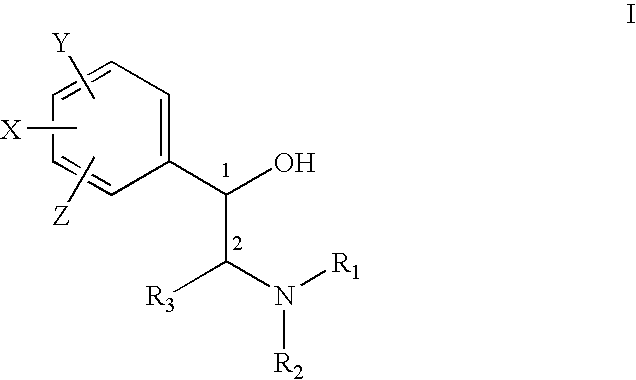
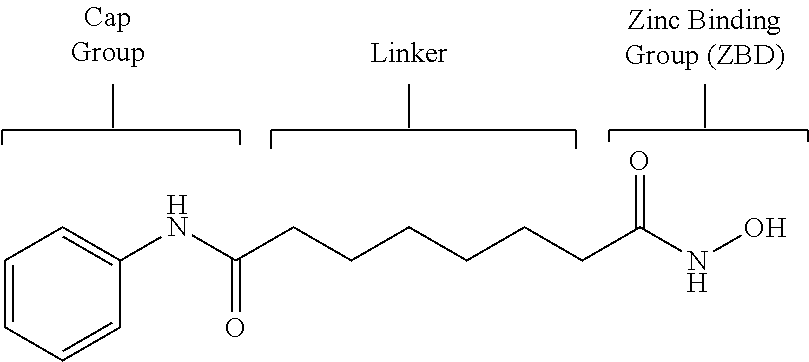
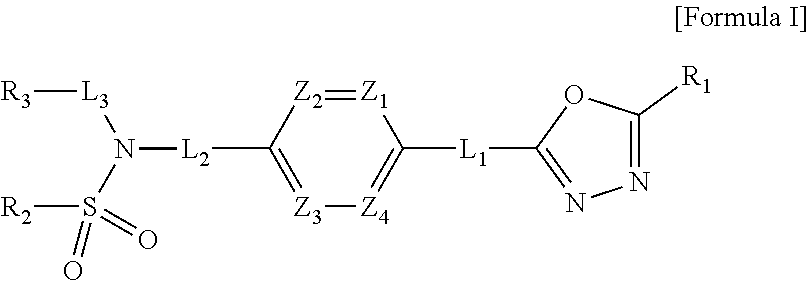
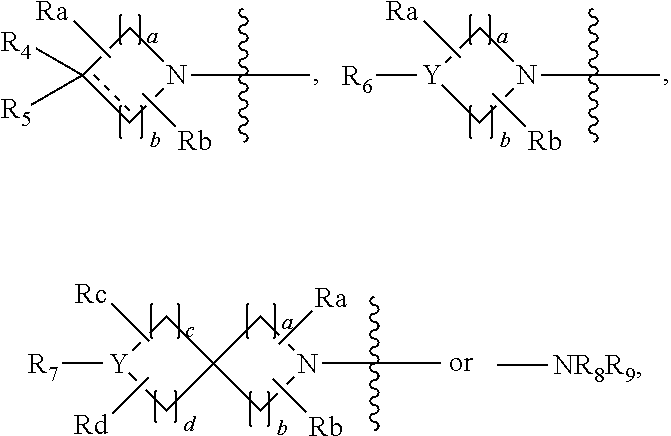
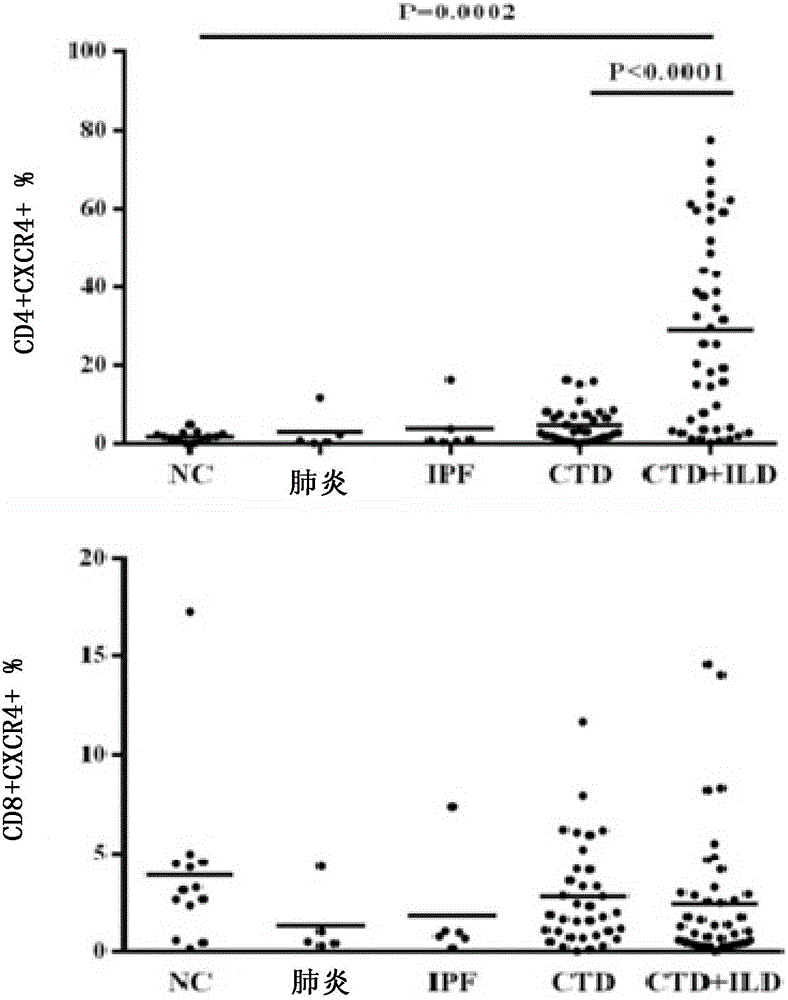
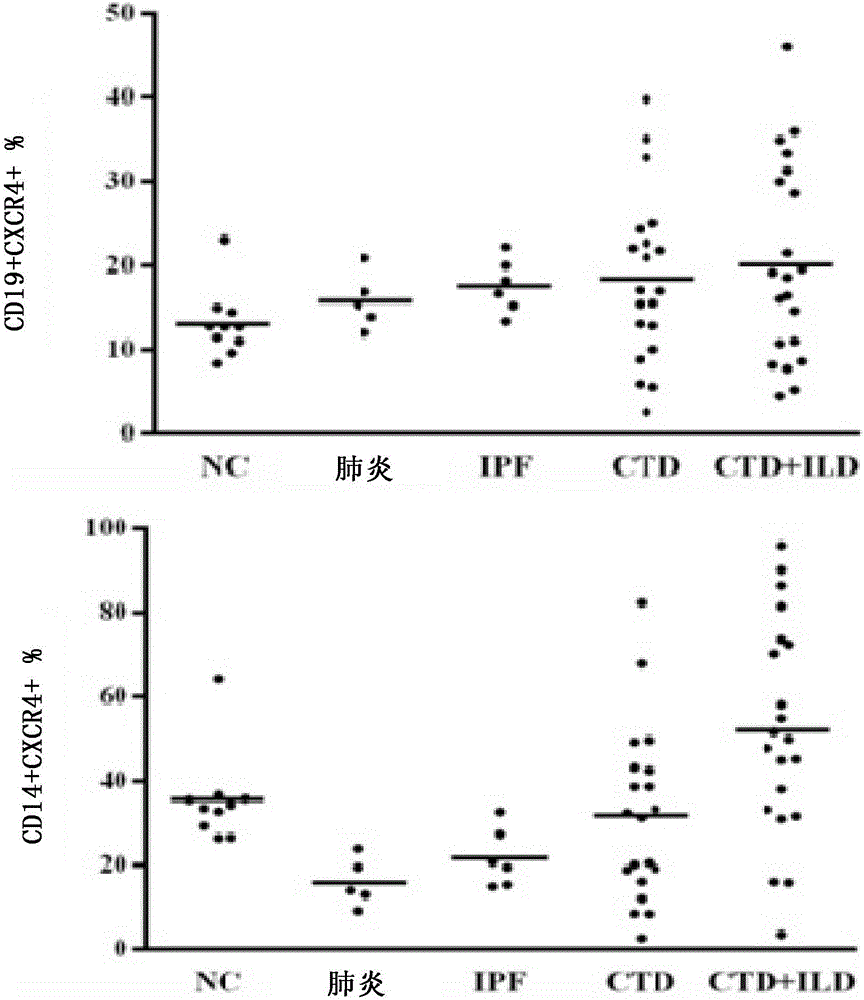
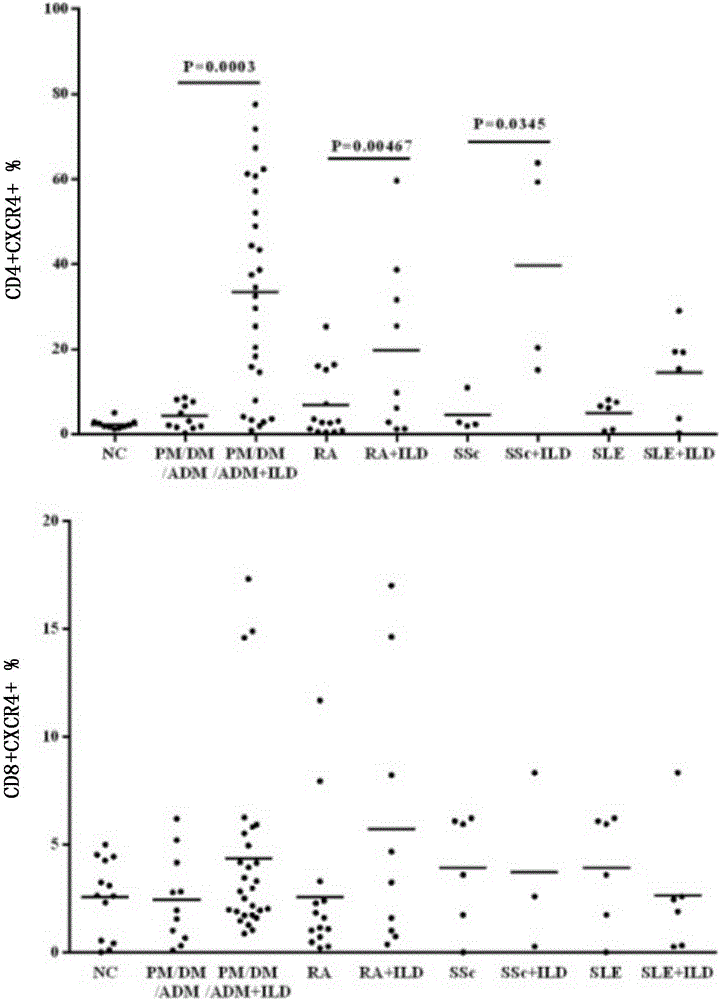
Patents
Literature
56 results about "Connective tissue disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A connective tissue disease (collagenosis) is any disease that has the connective tissues of the body as a target of pathology. Connective tissue is any type of biological tissue with an extensive extracellular matrix that supports, binds together, and protects organs. These tissues form a framework, or matrix, for the body, and are composed of two major structural protein molecules: collagen and elastin. There are many different types of collagen protein in each of the body's tissues. Elastin has the capability of stretching and returning to its original length—like a spring or rubber band. Elastin is the major component of ligaments (tissues that attach bone to bone) and skin. In patients with connective tissue disease, it is common for collagen and elastin to become injured by inflammation (ICT). Many connective tissue diseases feature abnormal immune system activity with inflammation in tissues as a result of an immune system that is directed against one's own body tissues (autoimmunity).
Pharmaceutical compositions
ActiveUS8268806B2Achieve beneficial effectAvoid their undesirable side effectOrganic active ingredientsMuscular disorderEstrogenic EffectsInsulin resistance
Owner:MYRIEL PHARM LLC
Minimal-heart-rate reduction parasympathetic stimulation
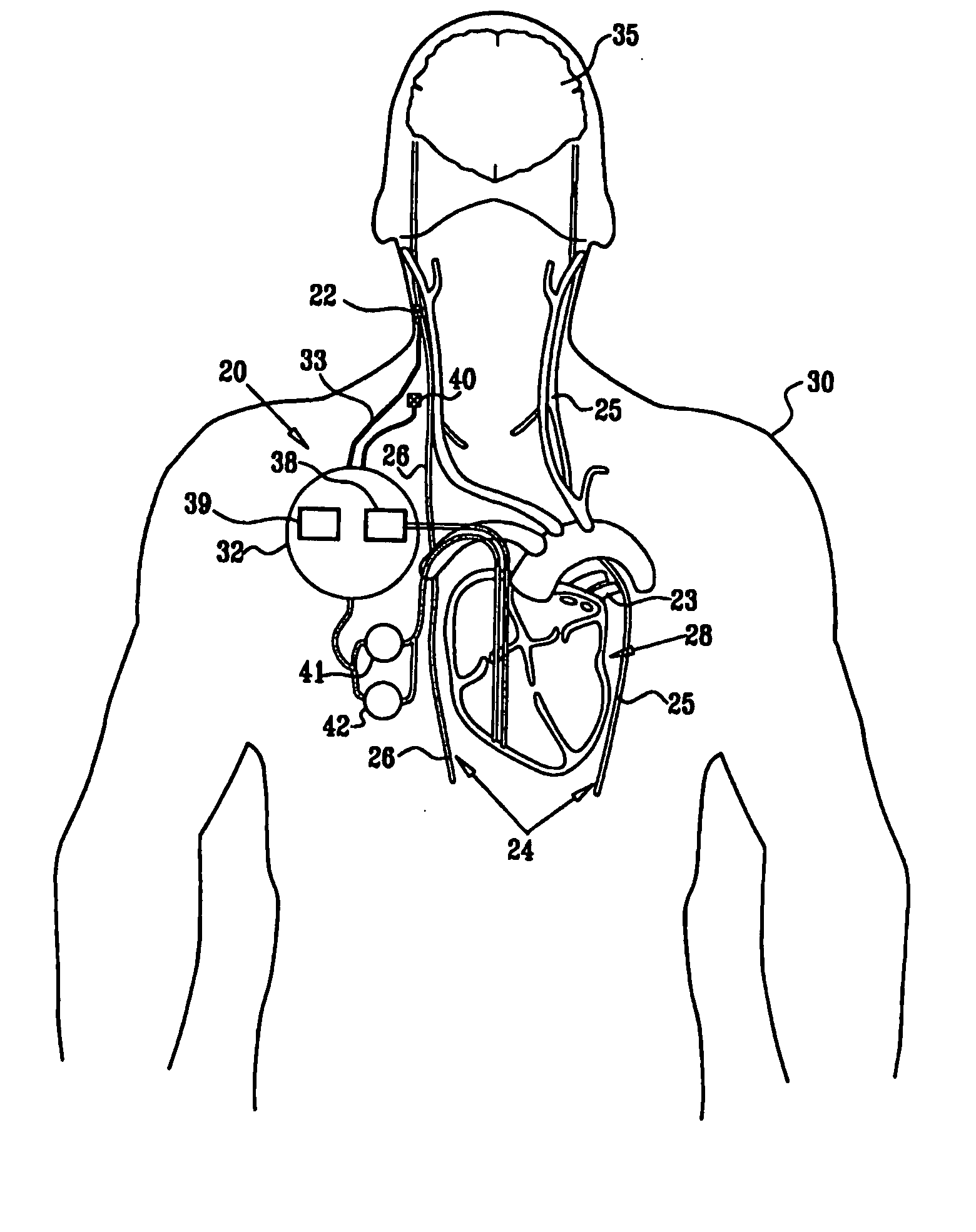
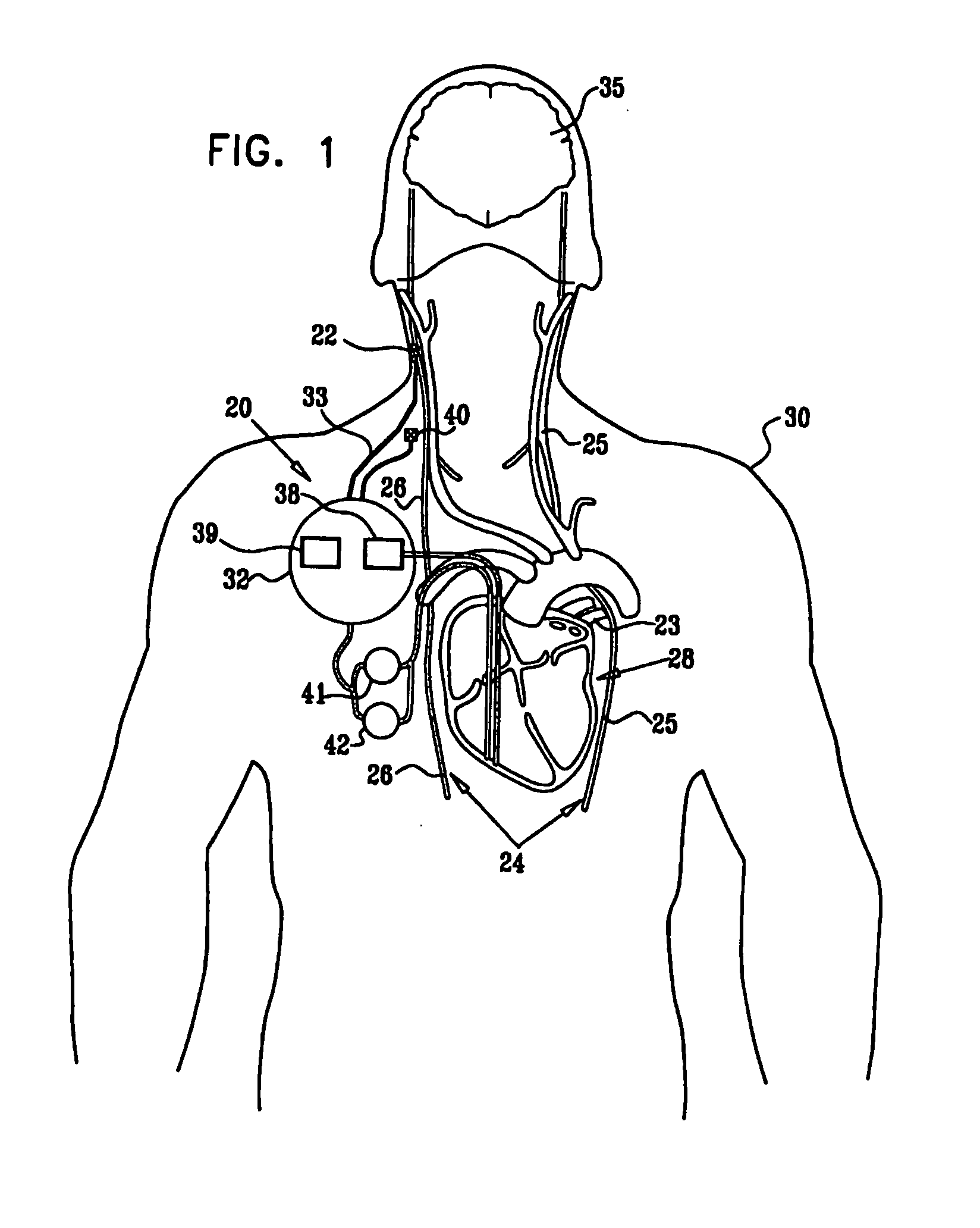
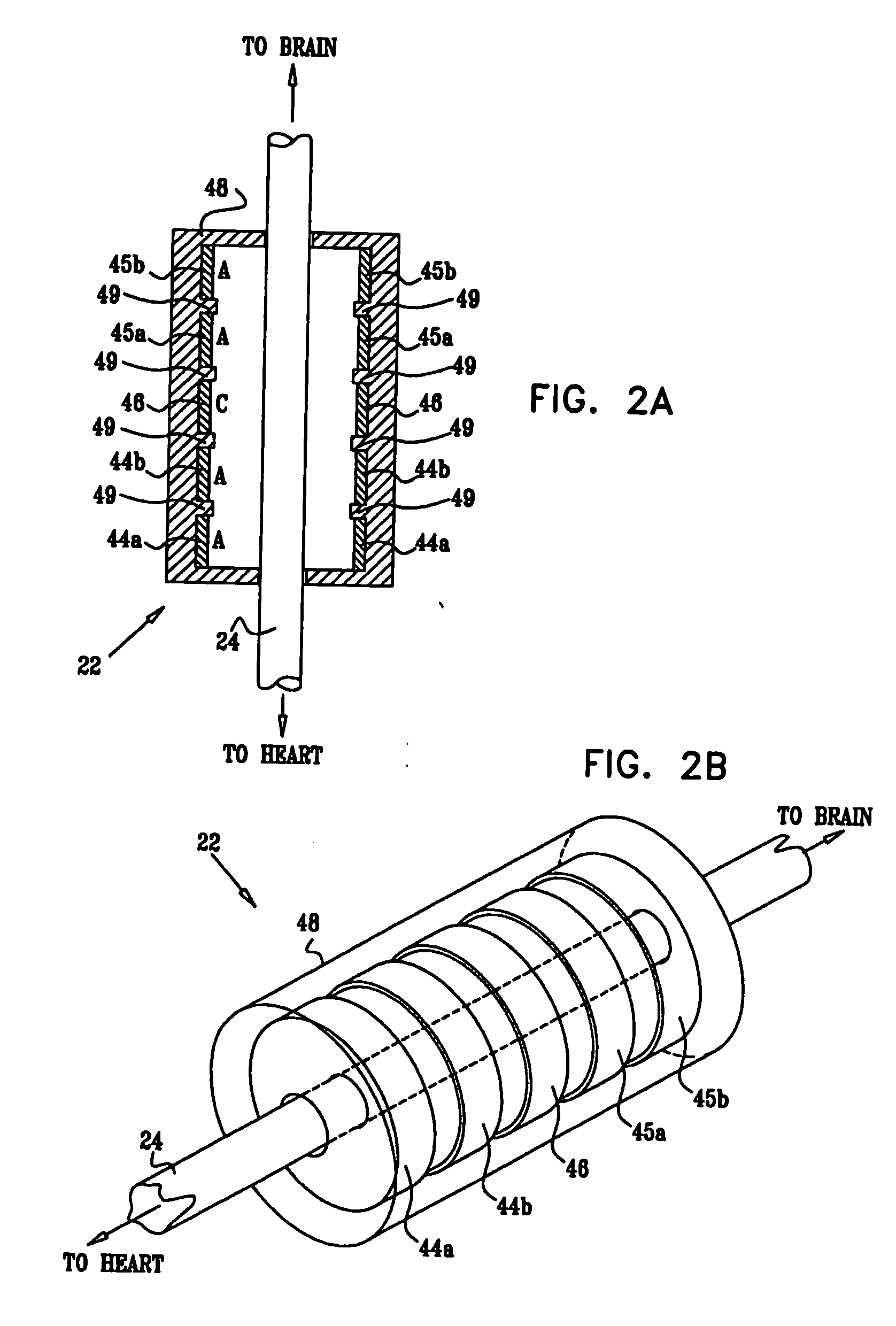
ActiveUS20080275514A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsInternal electrodesMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Methods for using JNK inhibitors for treating or preventing disease-related wasting
InactiveUS20040034084A1Inhibit progressPrevent worseningAntibacterial agentsBiocideChronic inflammatory diseaseKidney Failures
The present invention relates to methods useful for the treatment or prevention of disease-related wasting. The methods of the invention comprise the administration of an effective amount of a JNK Inhibitor. In one embodiment, the disease is HIV, AIDS, cancer, end-stage renal disease, kidney failure, chronic heart disease, obstructive pulmonary disease or tuberculosis. The methods can further comprise the administration of a therapeutic or prophylactic agent useful for the treatment or prevention of HIV, AIDS, cancer, end-stage renal disease, kidney failure, chronic heart disease, obstructive pulmonary disease, chronic infectious diseases (e.g., osteoarthritis and bacterial endocarditis), chronic inflammatory diseases (e.g., scleroderma and mixed connective tissue disease) or tuberculosis.
Owner:CELGENE CORP
Pharmaceutical compositions
ActiveUS20090054383A1Achieve beneficial effectPrevent adverse side effectsBiocideOrganic active ingredientsDiseaseEstrogenic Effects
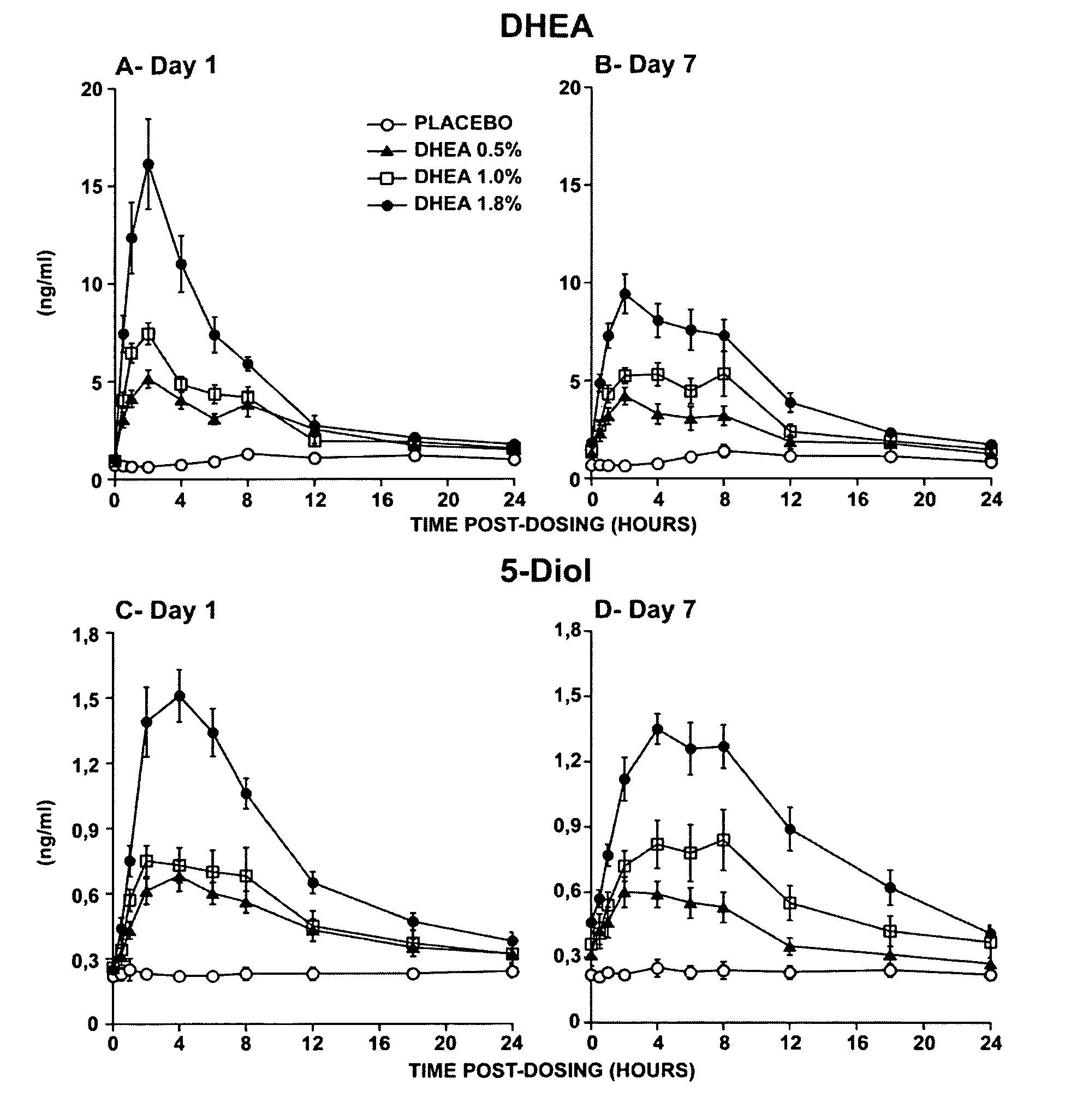
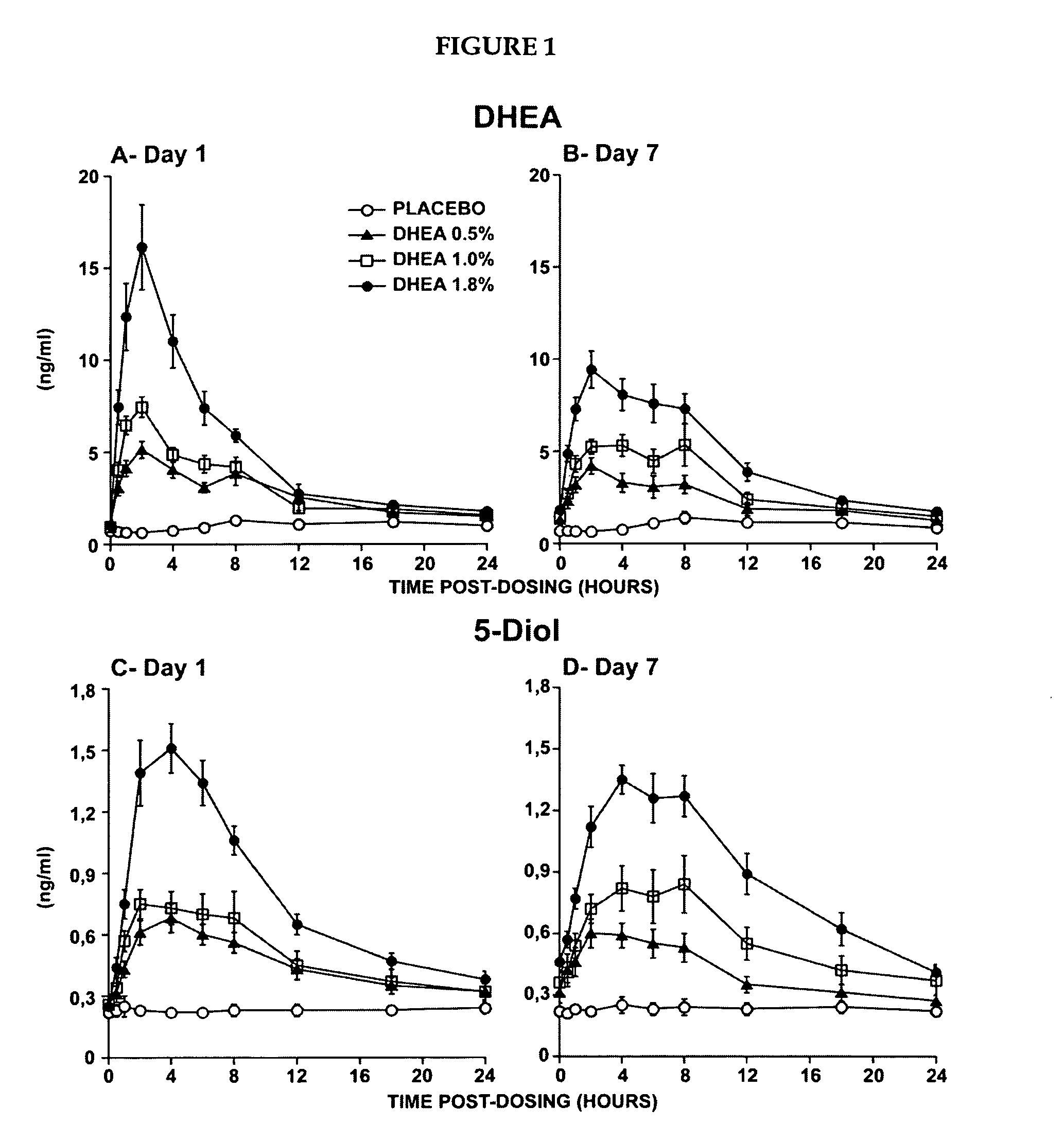
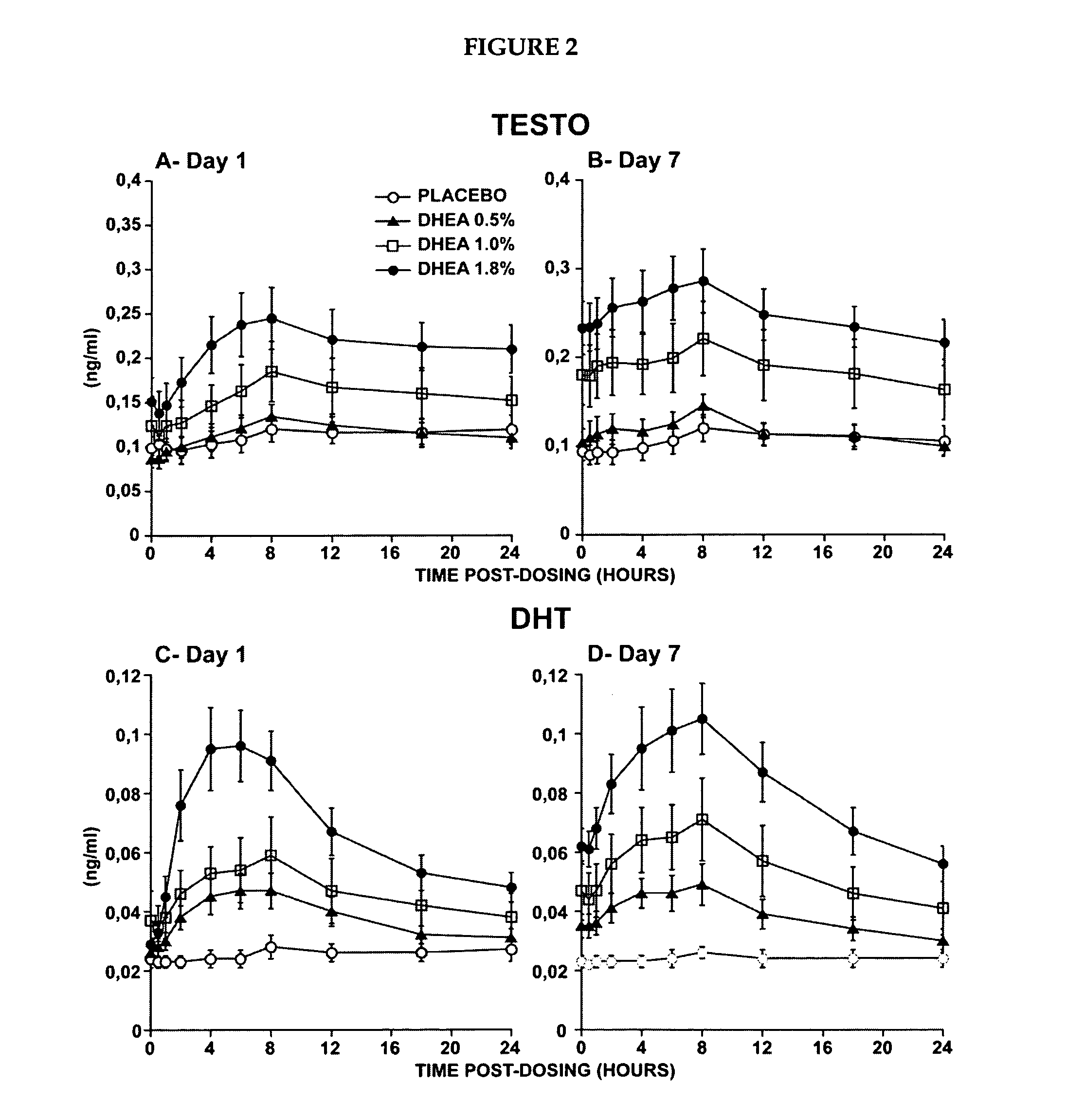
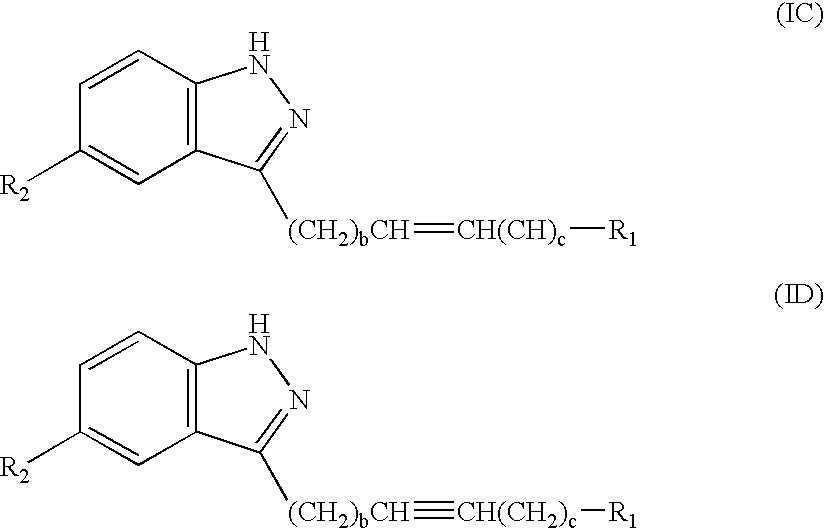
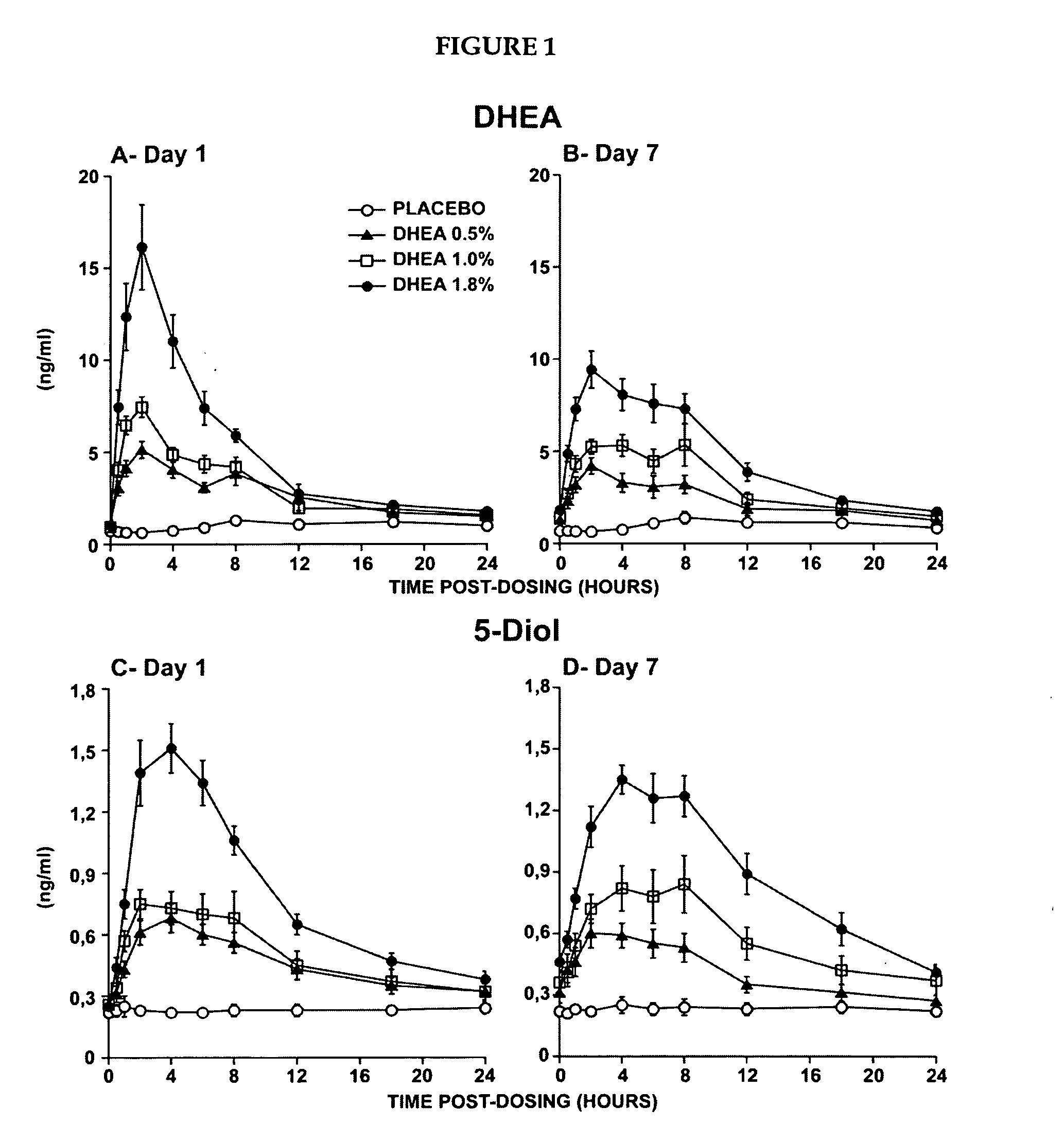
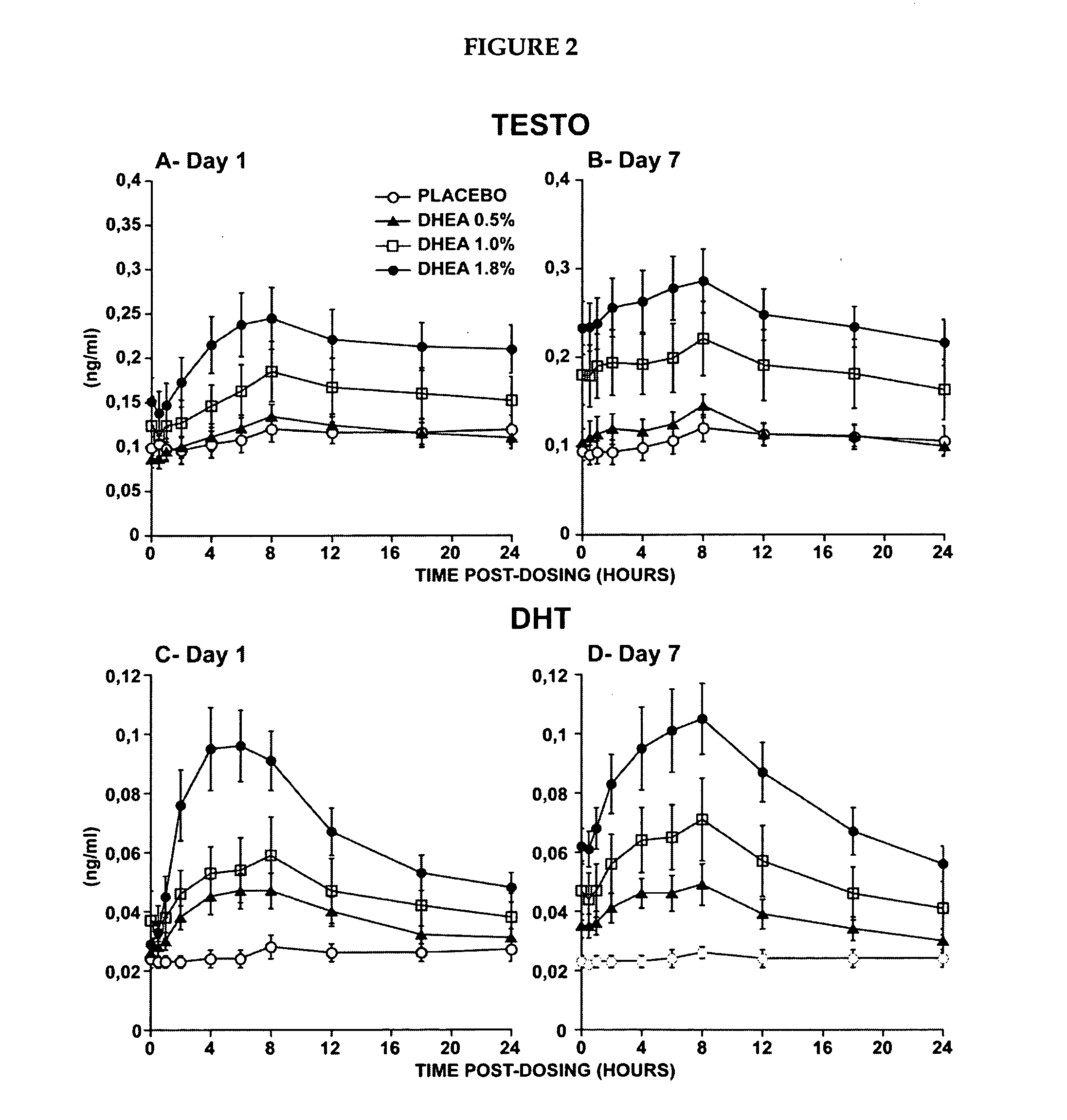
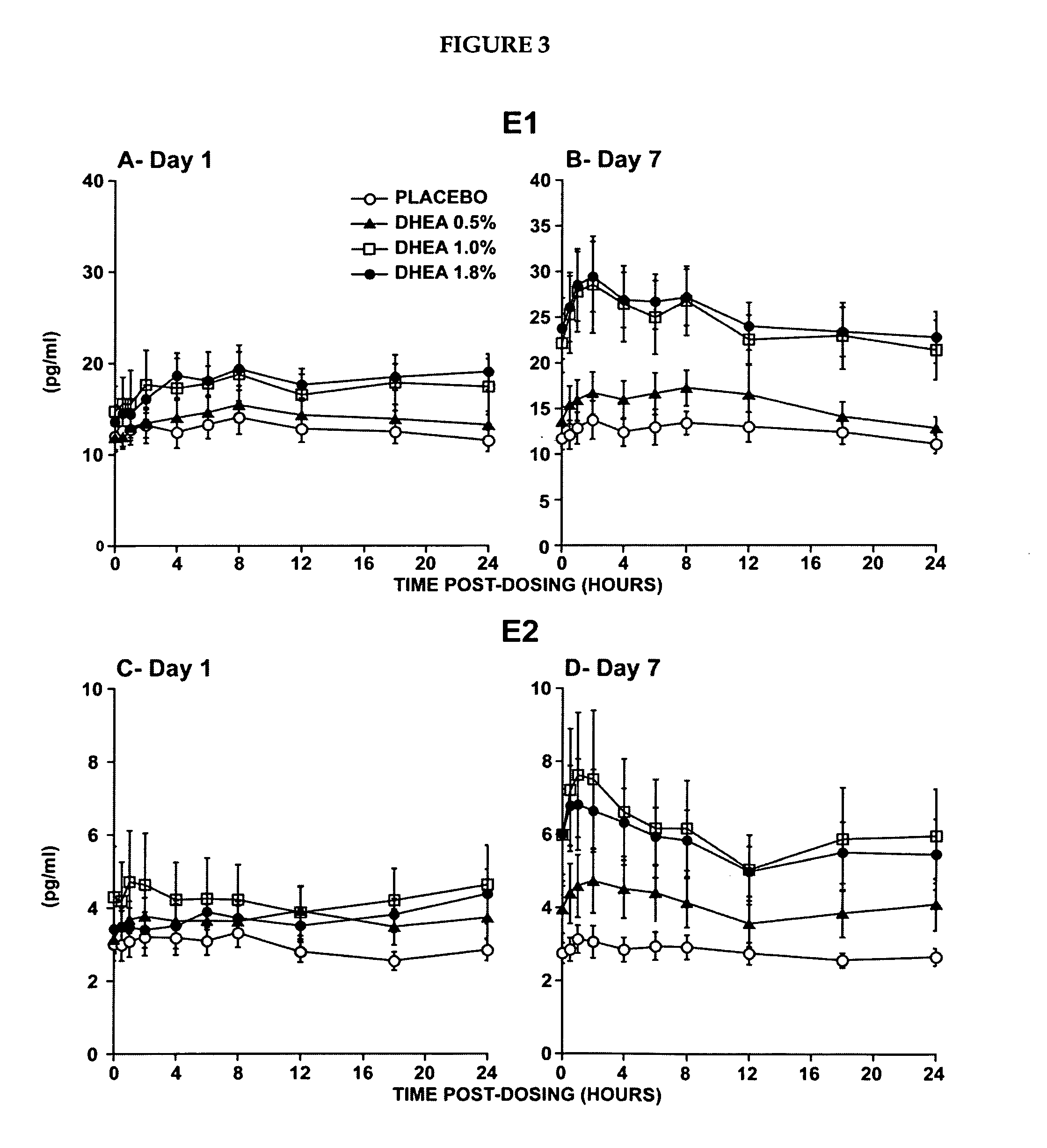
Novel methods for treating or reducing the likelihood of acquiring symptoms or diseases due to the menopause, in postmenopausal women, particularly osteoporosis, vaginal atrophy and dryness, hypogonadism, diminished libido, skin atrophy, connective tissue disease, urinary incontinence, breast, endometrial, ovarian and uterine cancers, hot flashes, loss of muscle mass, insulin resistance, fatigue, loss of energy, aging, physical symptoms of menopause, in susceptible warm-blooded animals including humans involving administration of a sex steroid precursor are disclosed. Said method comprising novel ways of administering and dosing dehydroepiandrosterone (DHEA) in order to take advantage of positive androgenic effects in the vaginal layers lamina propia and / or the layer muscularis, without undesirably causing systemic estrogenic effects in order to avoid the risk of breast and uterine cancer. Pharmaceutical compositions for delivery of active ingredient(s) useful to the invention are also disclosed.
Owner:MYRIEL PHARM LLC
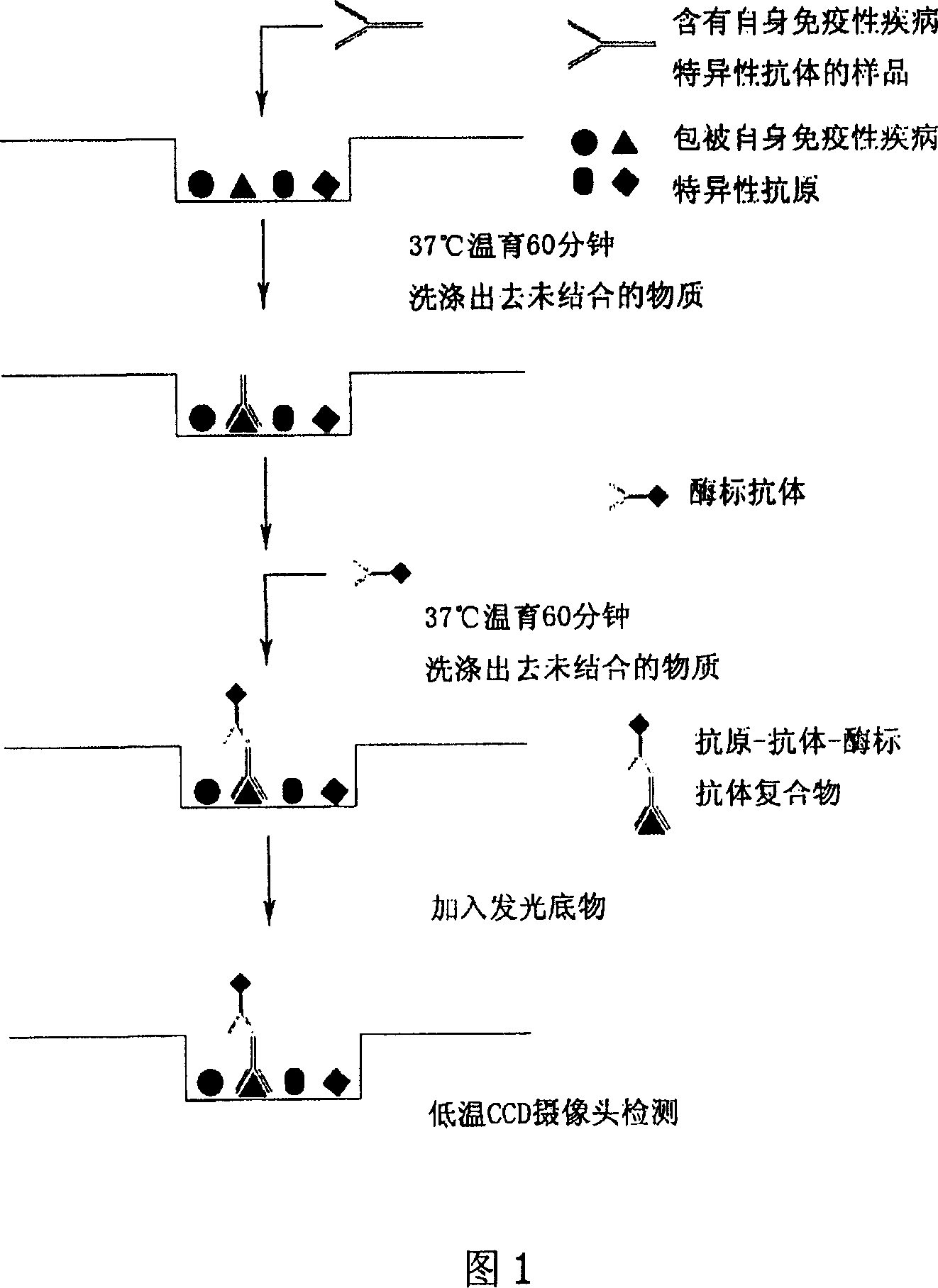
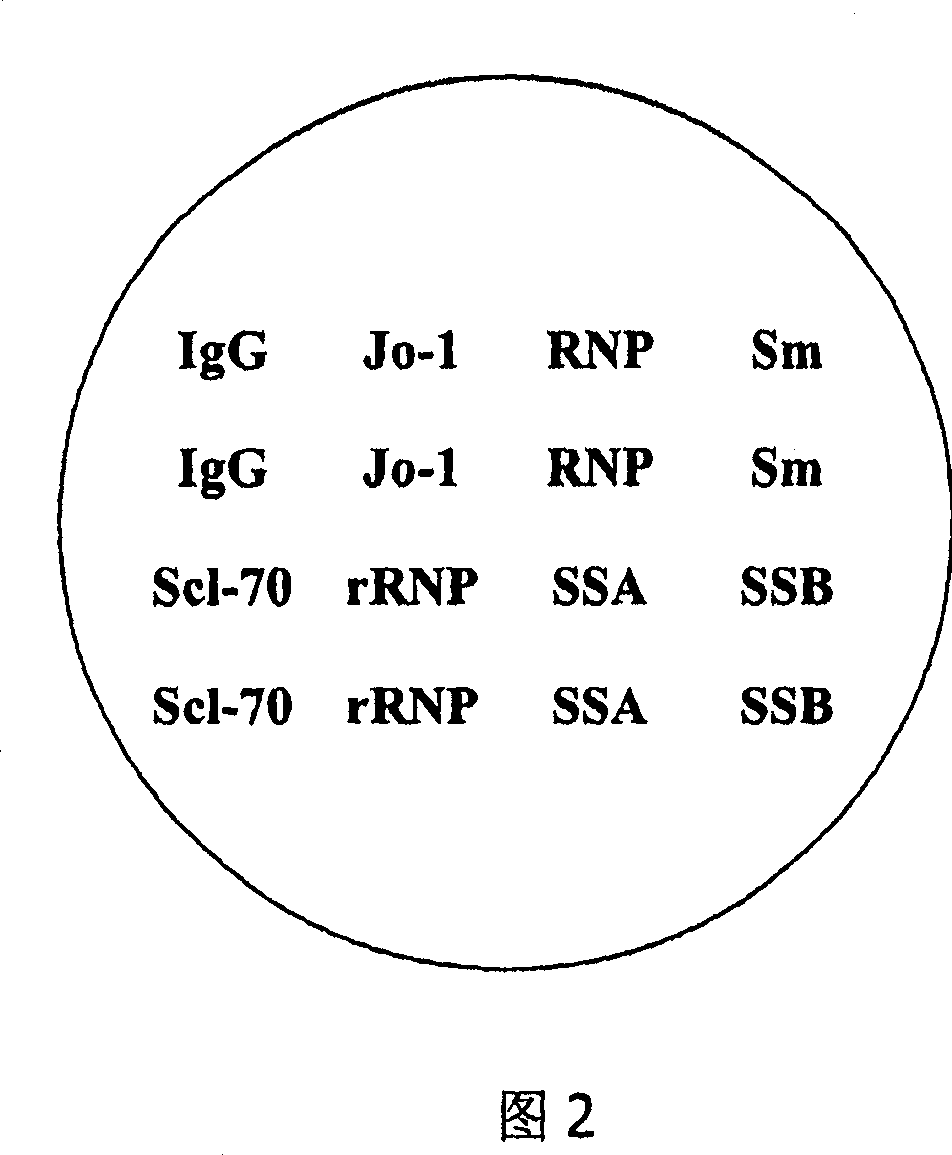
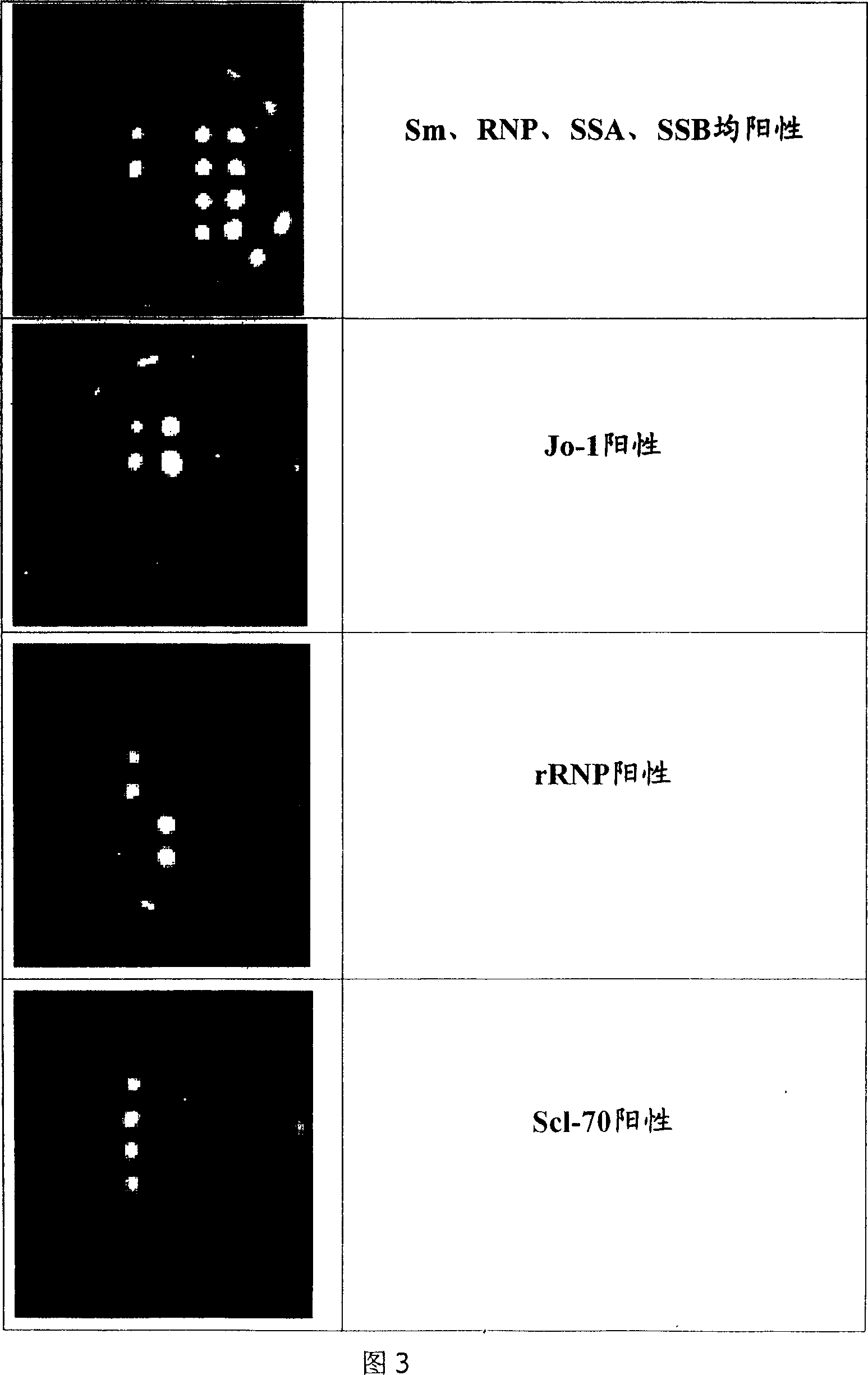
Microarray-ELISA detecting reagent kit for detecting autoimmunity disease relevant antibody spectrum
InactiveCN101063680AImprove throughputHigh parallelMaterial analysisDiffuse sclerodermaAutoimmune responses
This invention relates to one anti-extracting nuclear antigen spectrum array to ELISA test agent case for selecting for systematic lupus erythematosus, mixed connective tissue disease, Sjogren syndrome, systemic scleroderma, polymyositis and atrophic arthritis system self immune property antigen ENA spectrum micro array to enzyme immune agent case.
Owner:BEIJING BGI GBI BIOTECH +4
Lupus preparation and new preparing method
The present invention relates to a Chinese medicine composition, and especially a kind of Chinese medicine composition for treating lupus erythematosus, systemic scieroderm, dermatomyositis, panniculitis, behcets disease and connective tissue disease and its preparation process. The Chinese medicine composition is preferably prepared into dripping pill and soft capsule.
Owner:FUKANGREN BIO PHARMA
Use of nitroxides in treating skin disease
Methods of treating inflammatory skin diseases such as rosacea, atopic dermatitis, contact dermatitis, drug eruptions, psoriasis, seborrheic dermatitis, connective tissue diseases, autoimmune disorders, urticaria or hives, and inflammation associated with skin infections by topical or systemic administration of a nitroxide containing compound are provided.
Owner:BERNSTEIN ERIC F
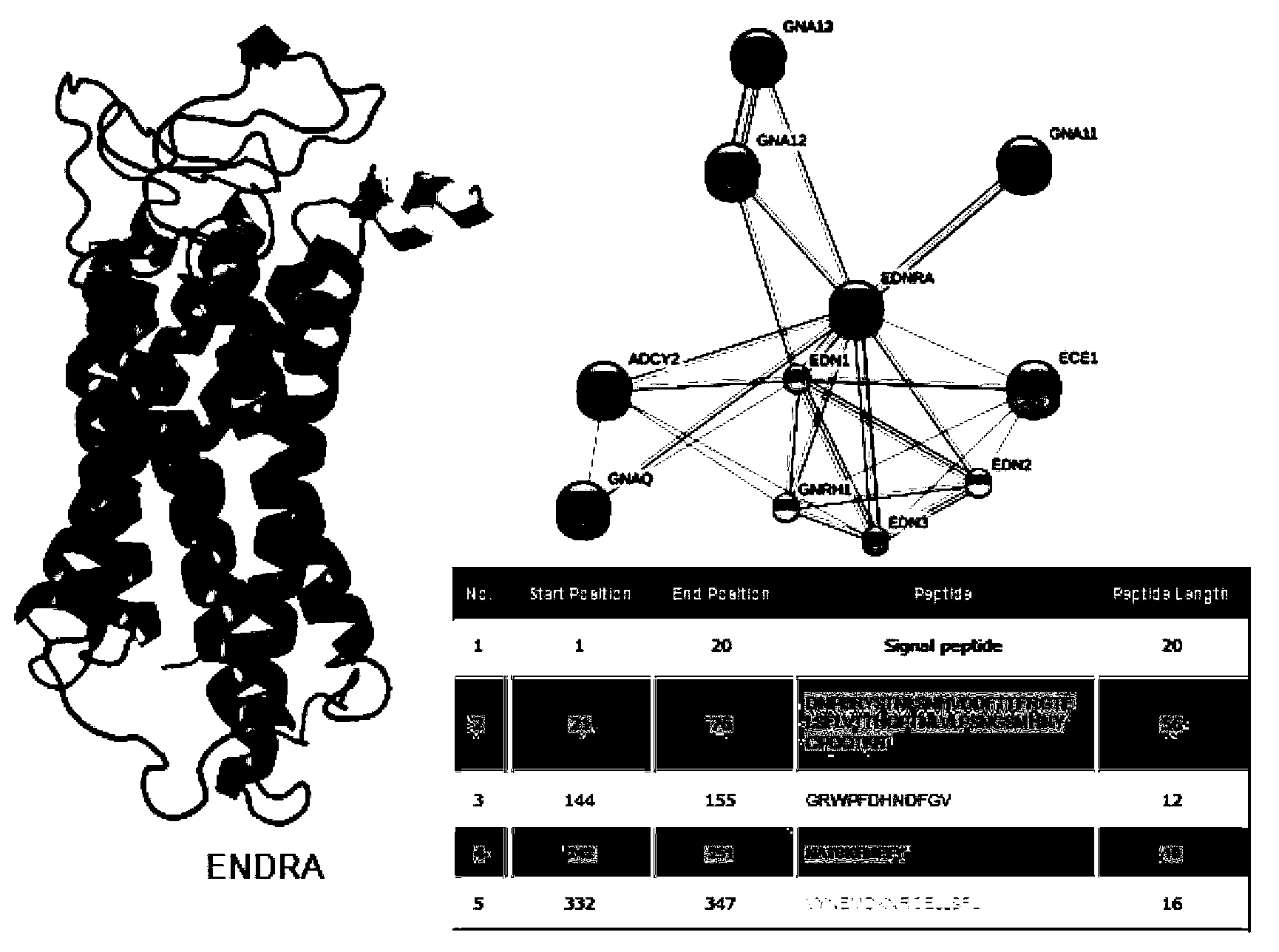
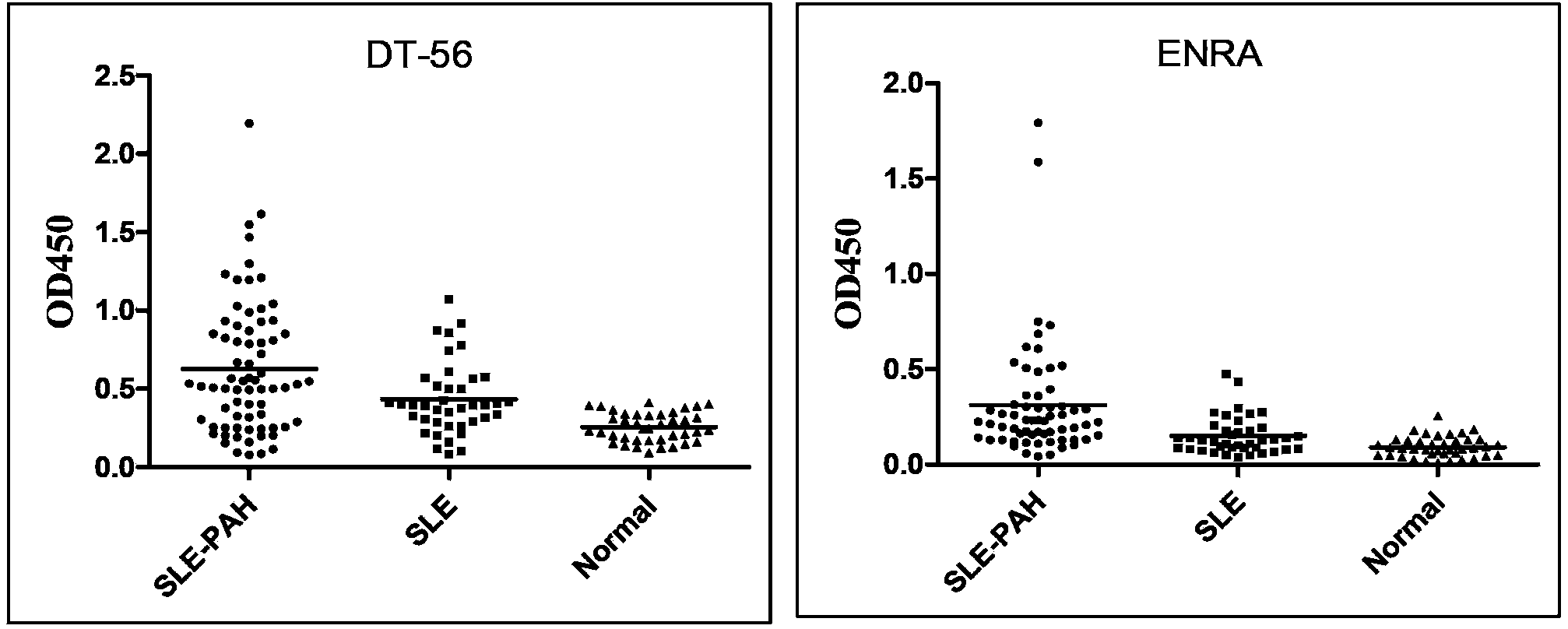
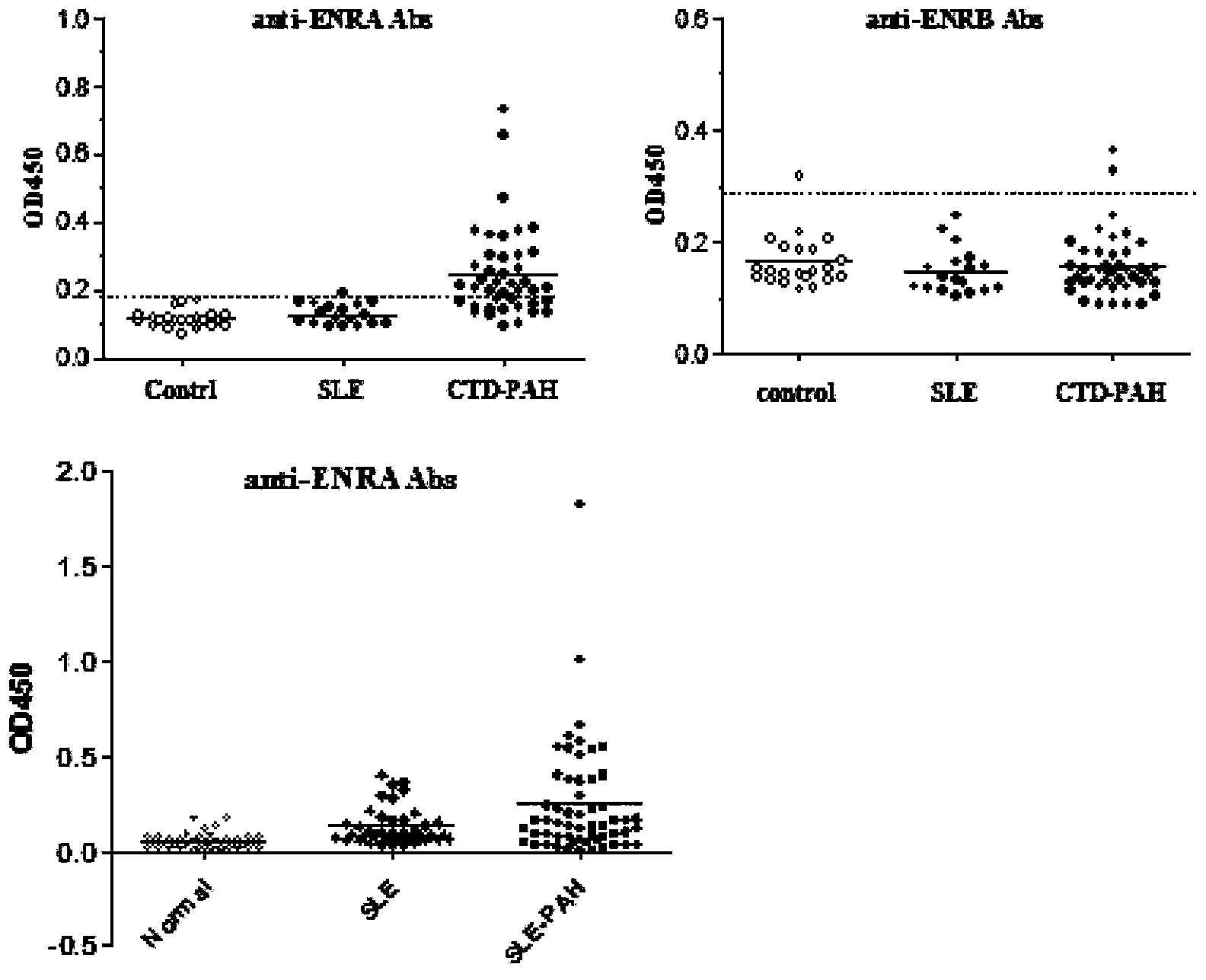
Enzyme-linked immunosorbent assay (ELISA) based on anti-ENRA (anti-endothelin receptor A) antibody of epitope antigen peptide and application thereof in CTD-PAH (connective tissue diseases-pulmonary arterial hypertension)
InactiveCN103728454ALow costImproving the practicality of clinical testingDisease diagnosisBiological testingAmino acidPulmonary hypertension
The invention relates to an enzyme-linked immunosorbent assay (ELISA) based on anti-ENRA (anti-endothelin receptor A) antibody of epitope antigen peptide; the enzyme-linked immunosorbent assay (ELISA) can be used in clinical tests of CTD-PAH (connective tissue diseases-pulmonary arterial hypertension); four extracellular peptide fragments with different lengths are synthesized in vitro, an epitope peptide fragment in good consistency with full-length ENRA is screened, the epitope peptide fragment is artificially synthesized to use as an antigen peptide package board to establish the enzyme-linked immunosorbent assay (ELISA) based on the anti-ENRA (anti-endothelin receptor A) antibody of the epitope antigen peptide; the epitope antigen peptide is a peptide fragment comprising the following amino acid sequence: DNPERYSTNLSNHVDDFTTFRGTELSFLVTTHQPTNLVLPSNGSMHNYCPQQTKIT; the enzyme-linked immunosorbent assay reduces the cost using full-length eukaryotic-expression endothelin receptor as a substrate, improves the clinical detection practicality, becomes a biomarker of CTD-PAH (especially SLE(systemic lupus erythematosus)-PAH), and provides valuable information for clinical diagnosis and treatment decisions.
Owner:吴庄民
Treatment of connective tissue diseases of the skin
ActiveUS20060235048A1Strong therapeutic potentialEasy to optimizeBiocideHydroxy compound active ingredientsDiseaseSkin sensitization
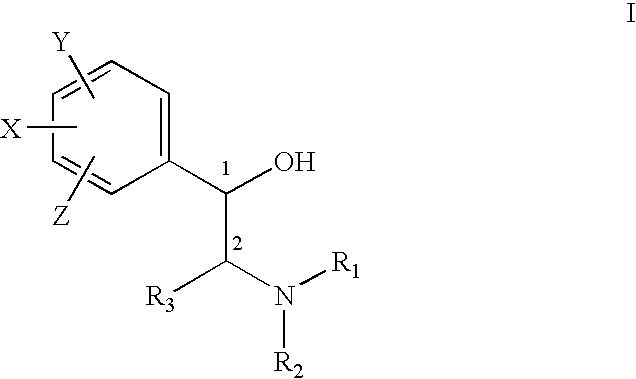
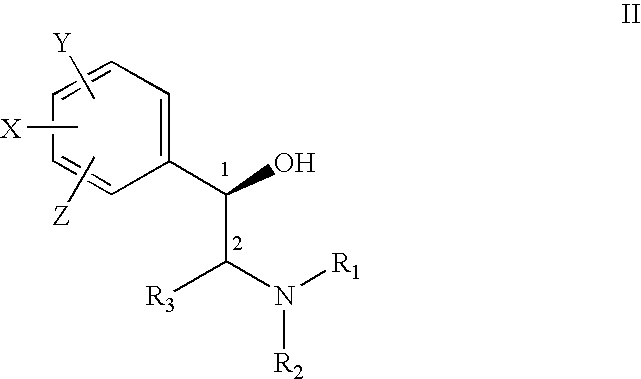
The present invention provides effective and safe medicaments for the treatment of connective tissue diseases of the skin, particularly with respect to the treatment of cutaneous forms of Lupus Erythematous. The medicaments comprise as the therapeutically active ingredient a beta2 adrenoceptor agonist. The invention furthermore relates to dermatological compositions without skin sensitization properties and which contain enantiomerically pure or enriched R-enantiomers of a beta2 adrenoceptor agonist.
Owner:ASTION PHARMA AS
Method for predicting and verifying the curative effect of glucocorticoid based on image omics
PendingCN110197236AOptimal penalty parameterIncrease success rateImage enhancementImage analysisInterstitial lung diseaseGlucocorticoid
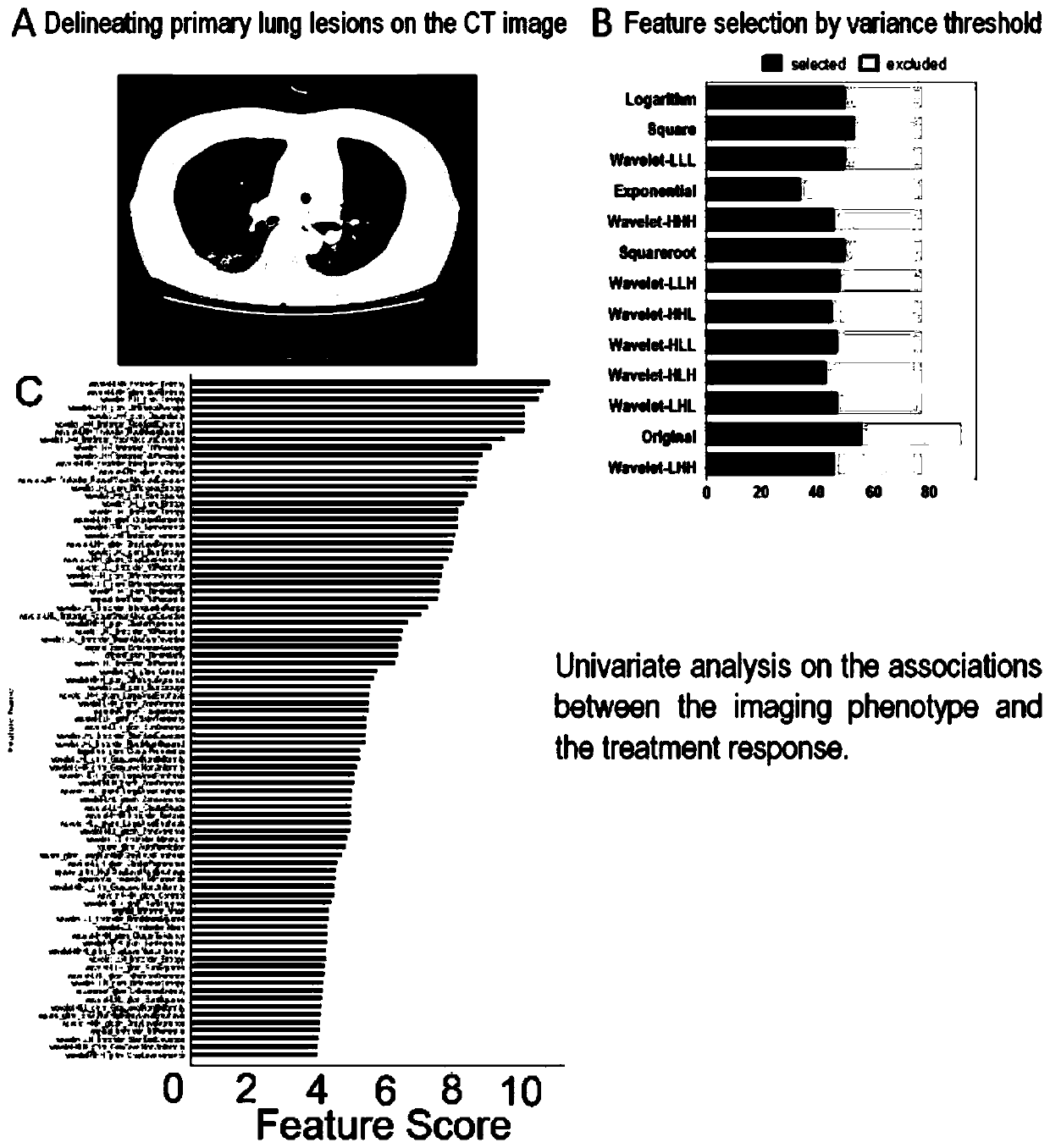
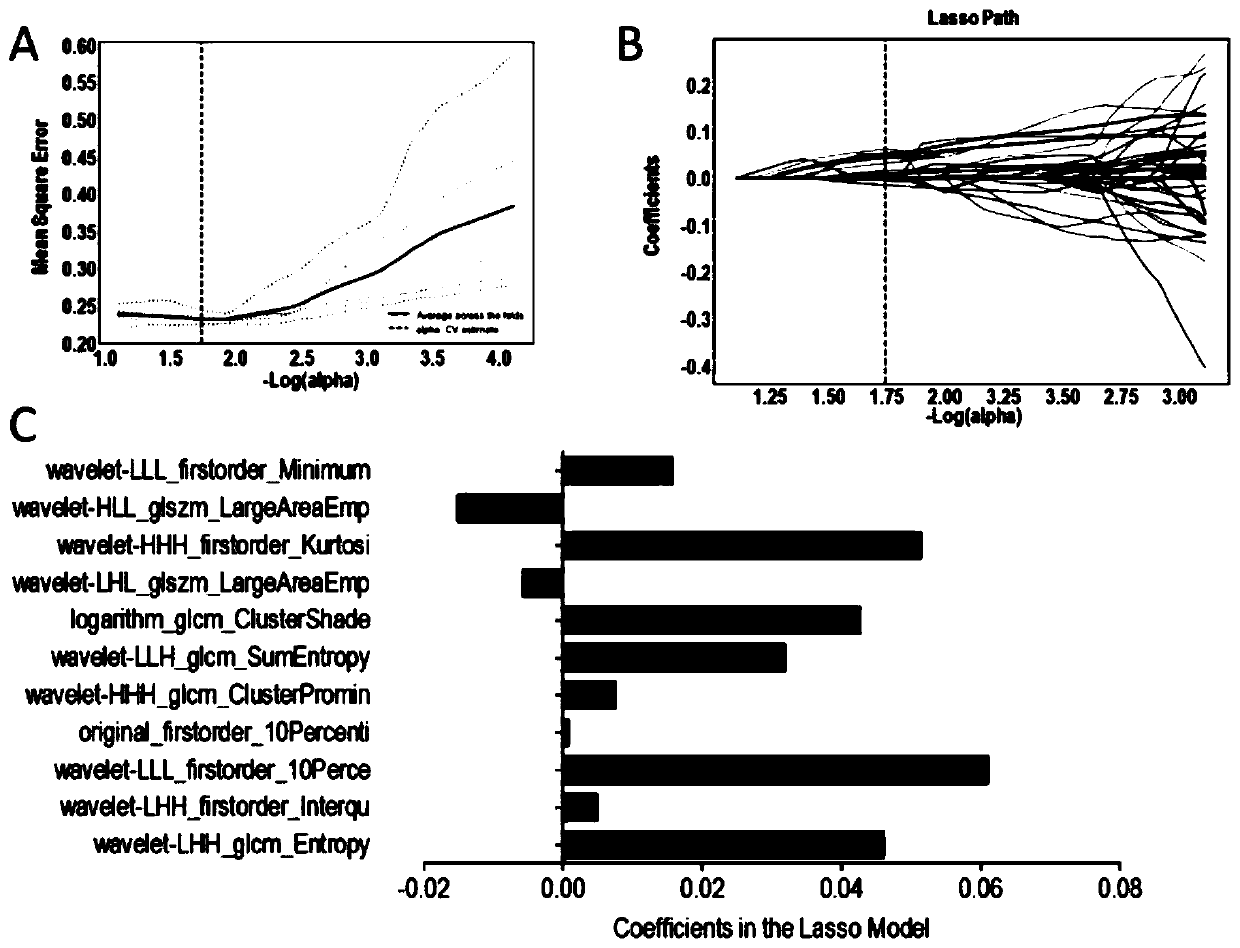
The invention provides a method for predicting and verifying the curative effect of glucocorticoid based on image omics, which comprises the following steps: S1, acquiring an original medical image ofa patient using glucocorticoid to treat connective tissue disease related interstitial pulmonary disease, and dividing the obtained original medical image into a training group and a verification group; s2, performing quantification processing on the original medical image to obtain image omics feature data; s3, establishing a prediction model of the image omics characteristics in the training group, and performing test verification in the verification group; and step S4, determining effective clinical characteristics and image omics characteristic tags adopting glucocorticoid, and performingverification in a verification group. According to the method, the curative effect of glucocorticoid is predicted and verified on the basis of imaging omics so as to identify patients sensitive to glucocorticoid treatment, and a specific reference basis is provided for doctors to diagnose and treat patients with connective tissue related interstitial pulmonary diseases, so that the patients are effectively treated, and the success rate of treatment is increased.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Preparation method of superparamagnetism conductive nano gamma-ferric oxide/ polyaniline-dexamethasone sodium phosphate
InactiveCN102000342AGood biocompatibilityImprove conductivityOrganic active ingredientsAntipyreticClasmatocyteMalignant lymphoma
The invention discloses a preparation method of superparamagnetism conductive nano gamma-ferric oxide / polyaniline-dexamethasone sodium phosphate, relating to the technical field of synthesis of superparamagnetism conductive nano gamma-ferric oxide / polyaniline-dexamethasone sodium phosphate. The superparamagnetism conductive nano gamma-ferric oxide / polyaniline-dexamethasone sodium phosphate is prepared by a doping method. The product of the invention can better suspend in water, still has favorable electrochemical activity and electric conduction magnetoconductivity within the pH range of human body environment, and can directionally reach diseased tissues under the regulation and control of an external magnetic field without damaging normal clasmatocyte. The targeted medicine is hopefully applied to treating acute leukemia, malignant lymphoma, connective tissue diseases, rheumatoid arthritis and the like. In addition, the method of the invention is simple, convenient, economical and favourable for industrial production and application.
Owner:YANGZHOU UNIV
Kit for detecting anti-moesin antibody
ActiveCN101929999AIncreased sensitivityImprove featuresDisease diagnosisColor/spectral properties measurementsEarly predictionMoesin
The invention discloses a kit for detecting an anti-moesin antibody. The kit comprises a solid-phase carrier and a moesin antigenic protein, wherein the moesin antigenic protein is a full-length human moesin. The kit can be applied to a method for early prediction and severity evaluation of connective tissue disease (CTD)-related lung involvement and evaluation is performed by mainly sampling a biological sample of a subject and detecting the quantity of the anti-moesin antibodies in the biological sample. The moesin is prepared by proteomics technology and gene recombination technology and an enzyme-linked immunosorbent assay (ELISA) detection kit for detecting the anti-moesin antibody is provided. The detectable rate of the kit for the CTD-related lung involvement is up to 51.7 percent.
Owner:SHANGHAI KEXIN BIOTECH
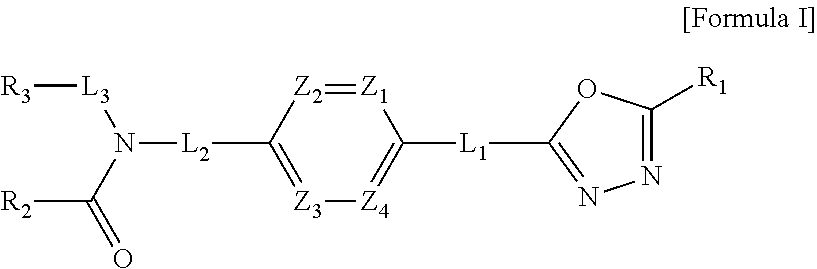
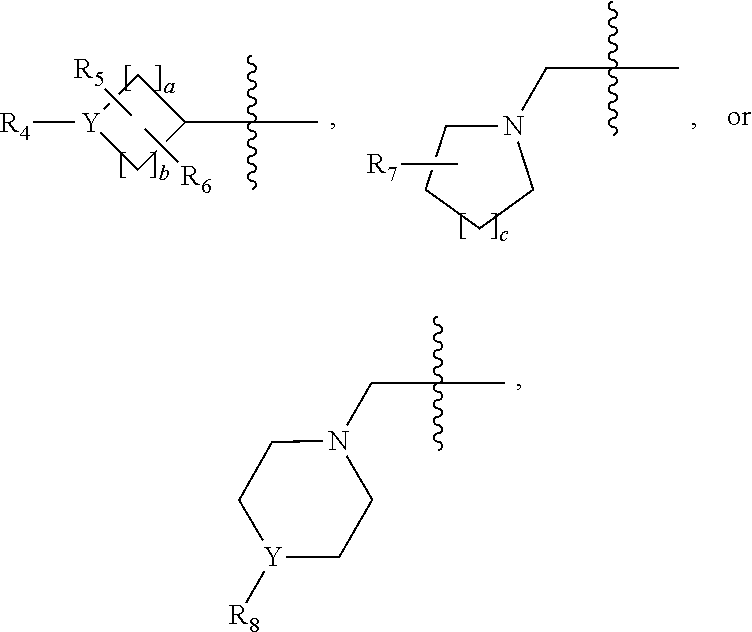
Oxadiazole amine derivative compounds as histone deacetylase 6 inhibitor, and the pharmaceutical composition comprising the same
ActiveUS20180273495A1Prevent goodConvenient treatmentOrganic active ingredientsSenses disorderNeoplasmNervous system
The present disclosure relates to novel compounds having histone deacetylase 6 (HDAC6) inhibitory activity, stereoisomers thereof or pharmaceutically acceptable salts thereof, the use thereof for the preparation of therapeutic medicaments, pharmaceutical compositions containing the same, a method for treating diseases using the composition, and methods for preparing the novel compounds. The novel compounds, stereoisomers thereof or pharmaceutically acceptable salts thereof according to the present disclosure have histone deacetylase (HDAC) inhibitory activity and are effective for the prevention or treatment of HDAC6-mediated diseases, including infectious diseases; neoplasms; endocrine, nutritional and metabolic diseases; mental and behavioral disorders; neurological diseases; diseases of the eye and adnexa; cardiovascular diseases; respiratory diseases; digestive diseases; diseases of the skin and subcutaneous tissue; diseases of the musculoskeletal system and connective tissue; or congenital malformations, deformations and chromosomal abnormalities.
Owner:CHONG KUN DANG PHARMA CORP
Inhibitors of matrix metalloproteinases
InactiveUS7144917B2Inhibit ovulationPrevent implantationBiocideOrganic chemistryAbnormal tissue growthAutoimmune responses
The invention provides compounds that inhibit MMPs; methods for treating or preventing cancer, angiogenesis, arthritis, connective tissue disease, cardiovascular disease, inflammation or autoimmune disease in a mammal; a method for inhibiting a matrix metalloproteinase in vivo or in vitro; and a method for imaging a tumor in vivo or in vitro.
Owner:WAYNE STATE UNIV
1,3,4-oxadiazole sulfamide derivative compounds as histone deacetylase 6 inhibitor, and the pharmaceutical composition comprising the same
ActiveUS10464911B2Prevent goodConvenient treatmentNervous disorderOrganic chemistryNervous systemNeoplasm
Owner:CHONG KUN DANG PHARMA CORP
Aminosugar and/or glycosaminoglycan composition having therapeutic use
An embodiment of the invention provides a composition having one or more active ingredients such as an aminosugar, more particularly, glucosamine, glucosamine salt, and mixtures thereof, and a glycosaminoglycan, more particularly, chondroitin, chondroitin salts, and mixtures thereof having a therapeutic use and is stable for at least 24 months. Additionally, the composition includes a diluent, glidant, and lubricant. The invention also provides a method of treating a connective tissue disease, injury, or condition comprising administering to a mammal any one of the compositions described herein. The composition can be in tablet or capsule form.
Owner:STC UNM
Predictive Biomarkers for Detection of Organ Damage in Autoimmune Illnesses and Other Diseases
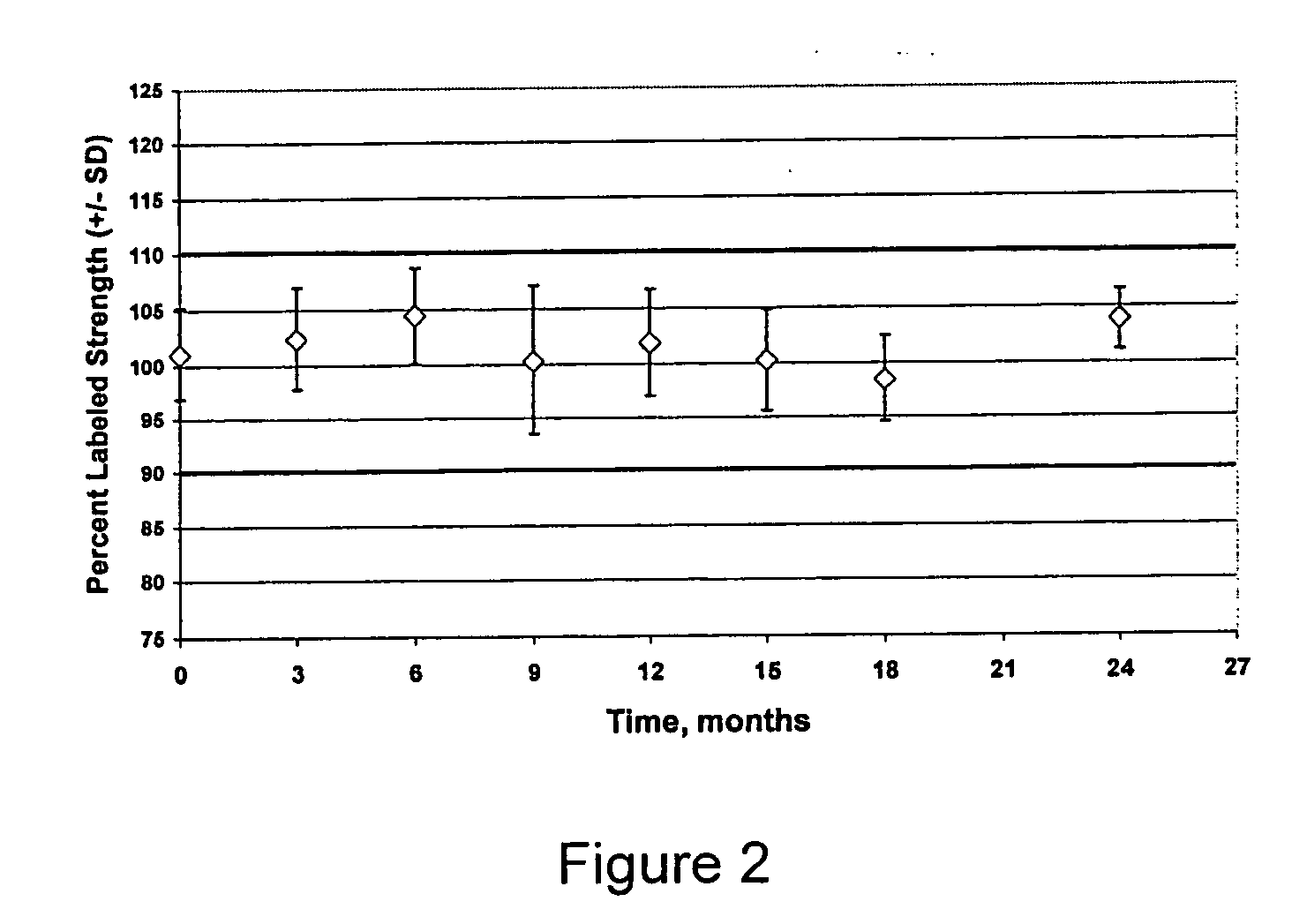
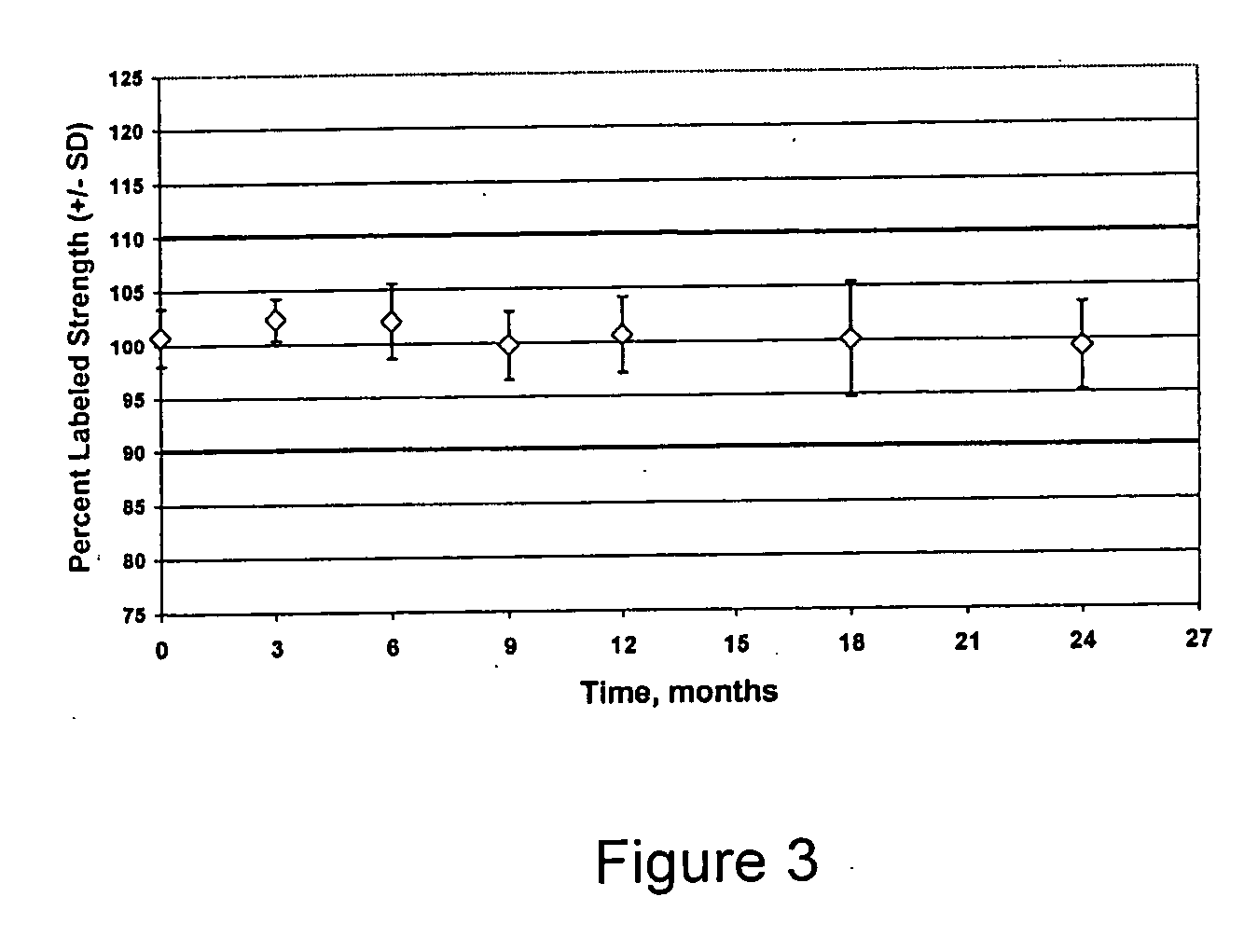
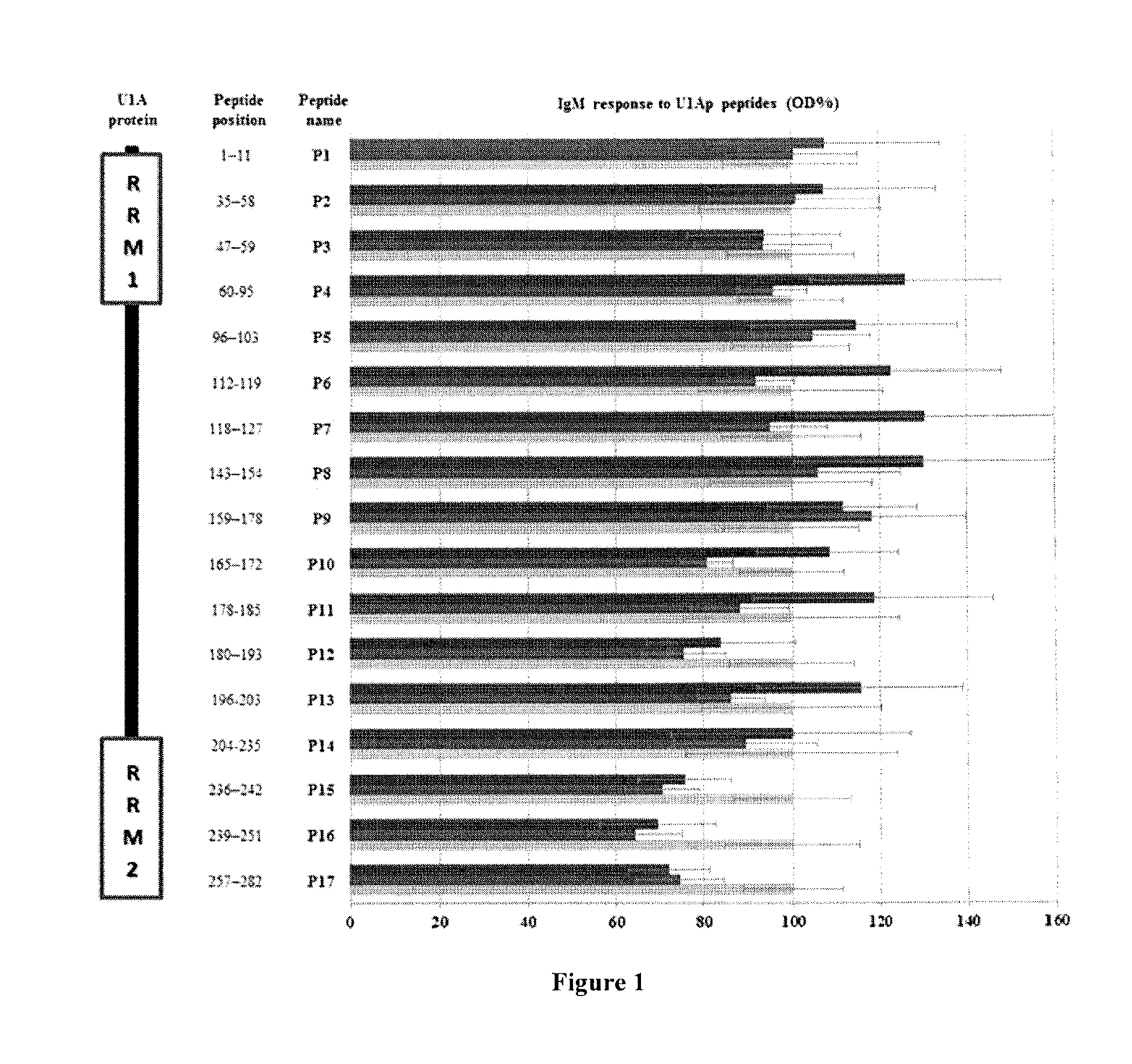
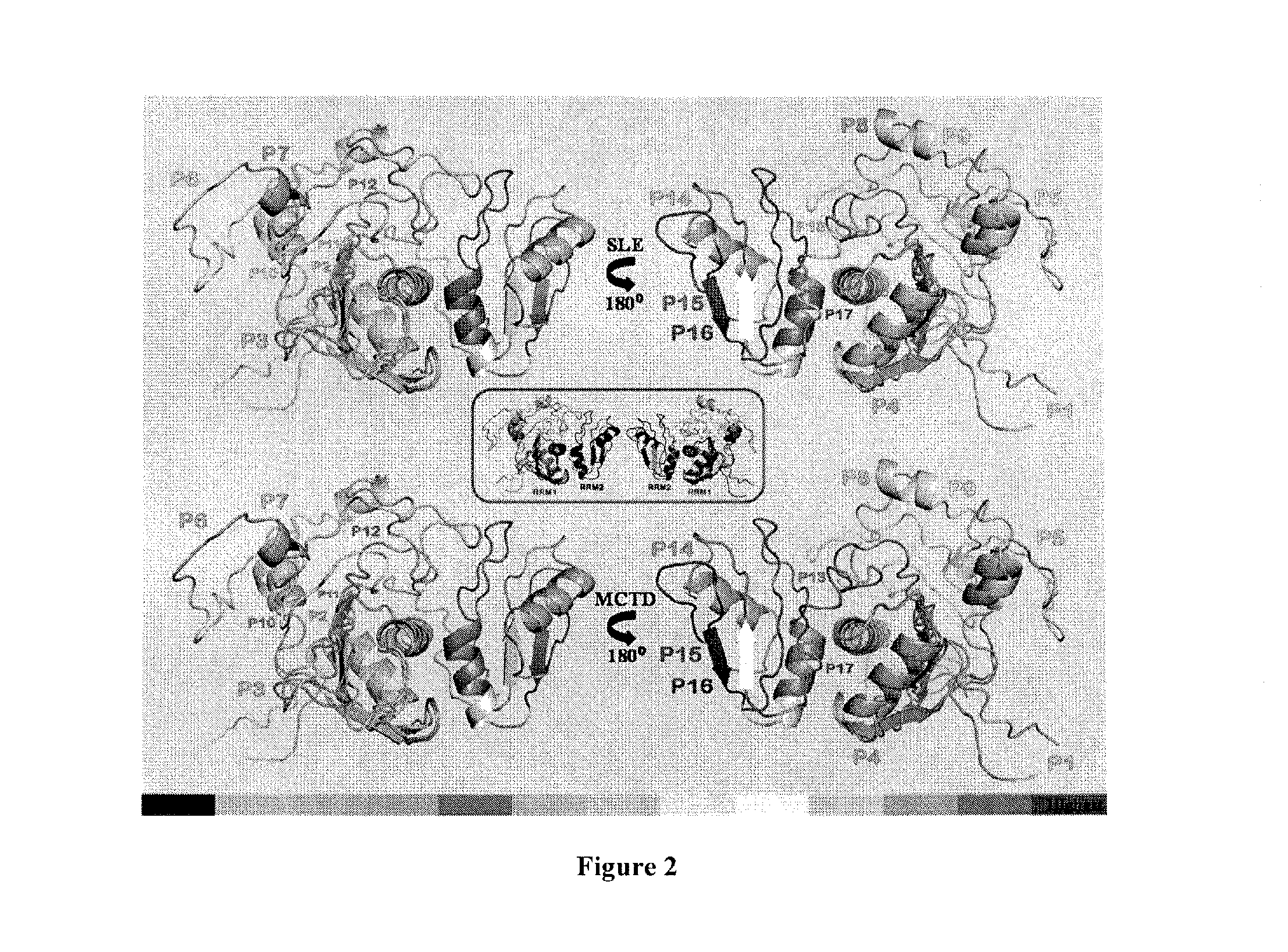
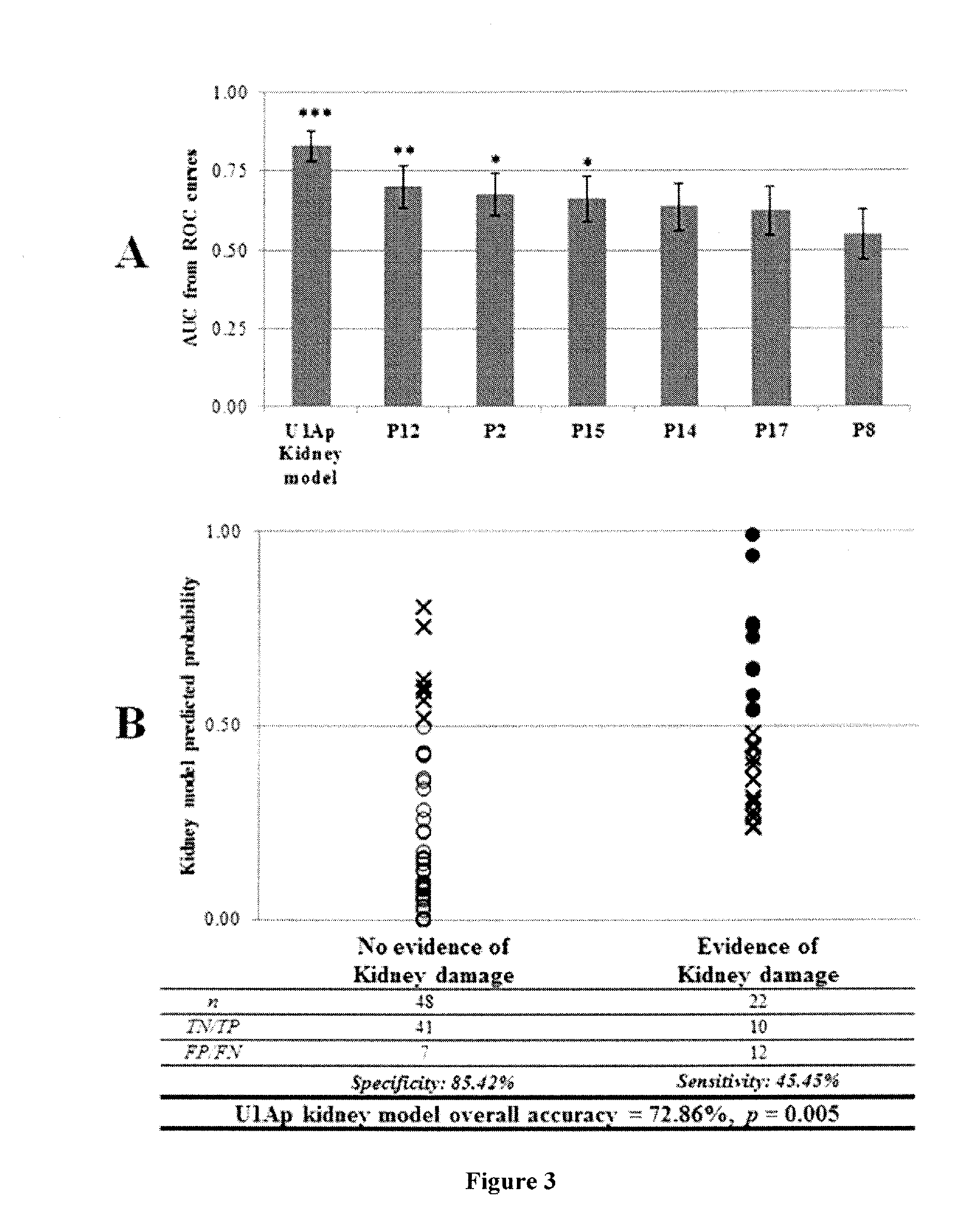
The invention provides methods for identifying the presence of, or an increased risk of developing, organ damage in a subject having an autoimmune disease, for example, Systemic Lupus Erythematosus (SLE) or Mixed Connective Tissue Disease (MCTD), or other disease in which the lungs and / or kidneys are involved. In one embodiment, a significantly increased combined IgM reactivity against the peptides having the sequences of SEQ ID NOs: 4, 9, 12, and 15 in a sample obtained from a patient compared to a healthy control indicates lung damage or an increased risk of developing lung damage in the subject. In another embodiment, a significantly increased combined IgM reactivity against the peptides having the sequences of SEQ ID NOs: 2, 8, 12, 14, 15, and 17, in a sample from a patient compared to a healthy control indicates kidney damage or an increased risk of developing kidney damage in the subject.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Platelet-rich plasma compositions
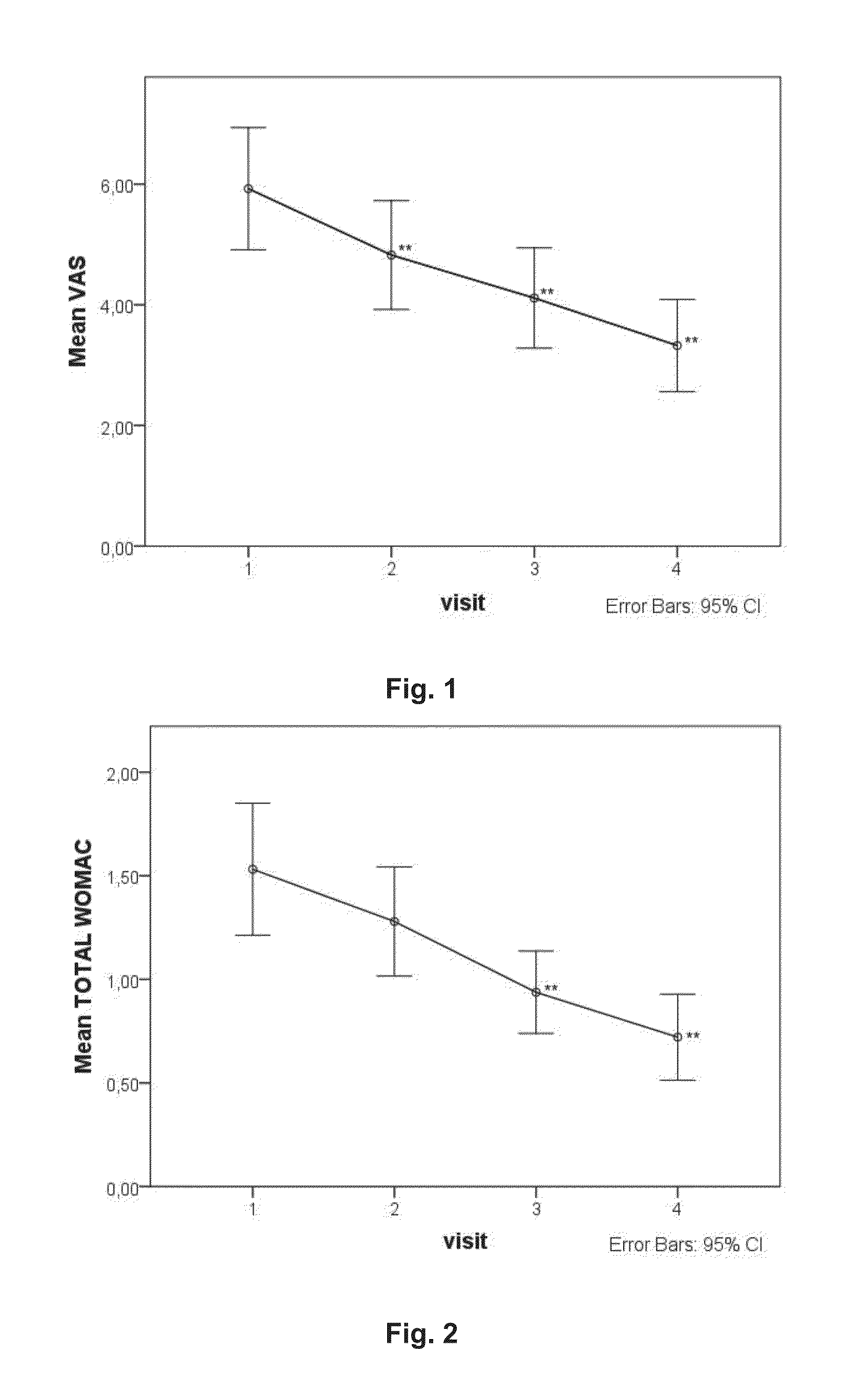
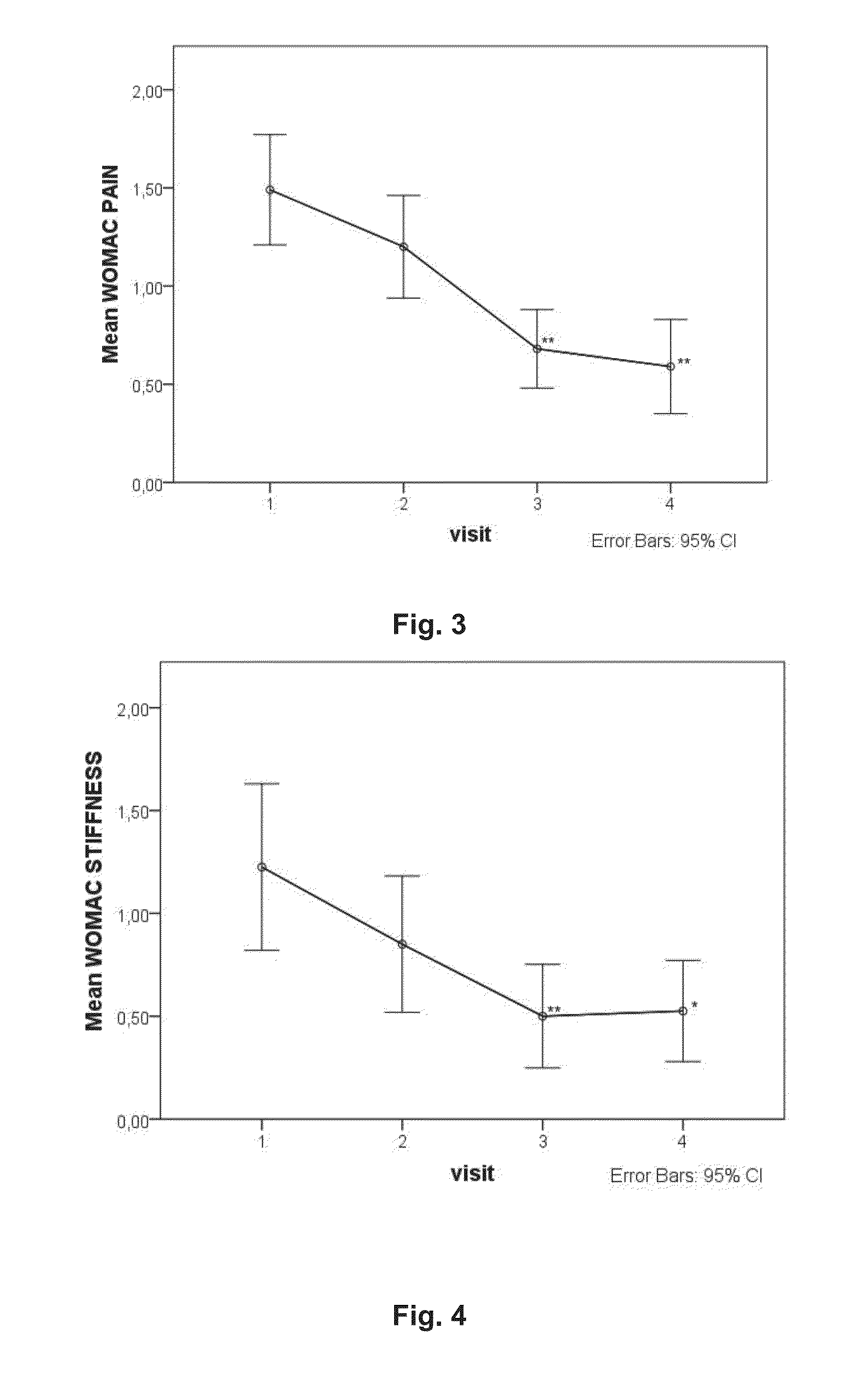
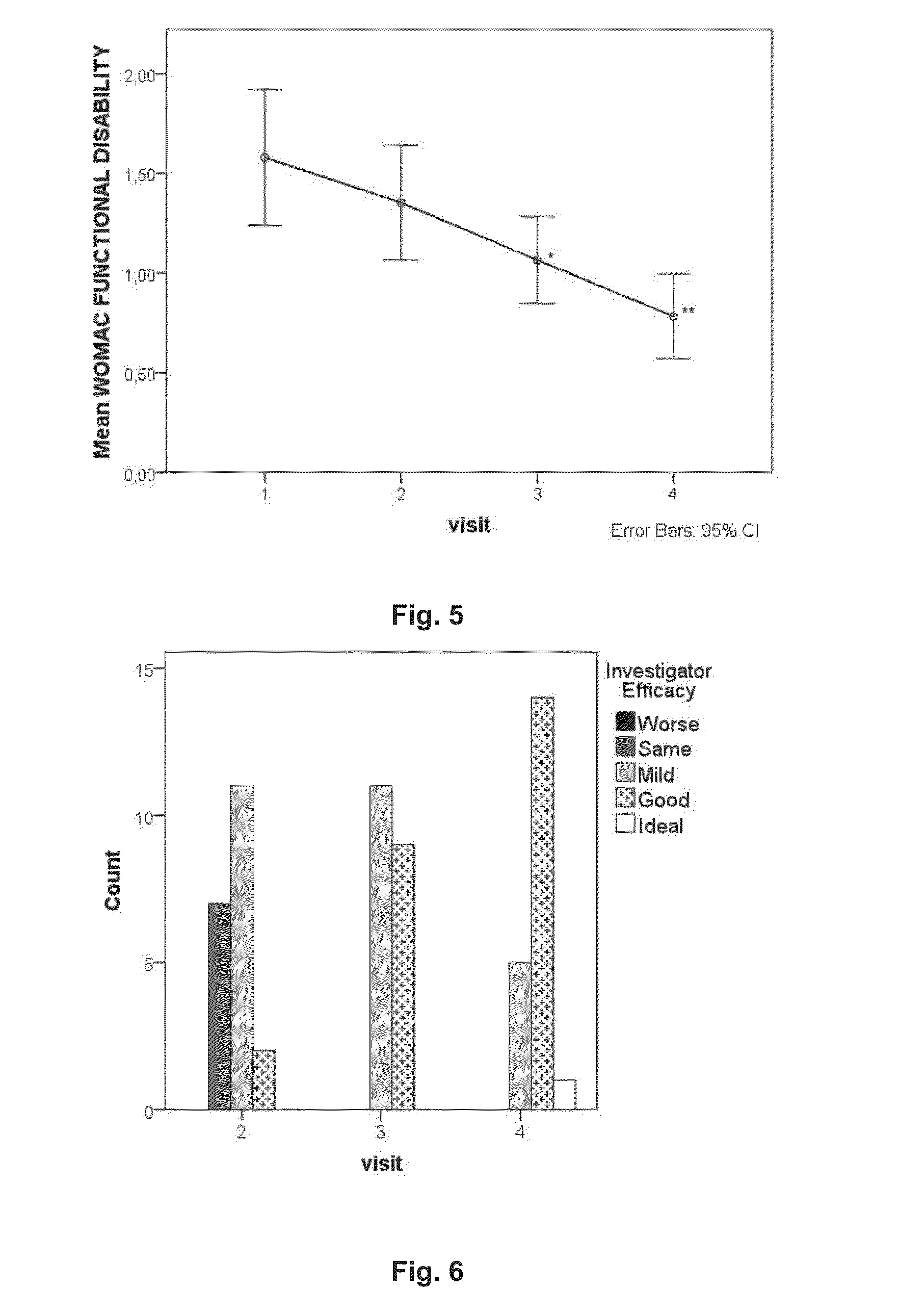
ActiveUS20130216626A1Effective in of painEffective preventionDispersion deliveryPeptide/protein ingredientsOral medicationConnective tissue
The present invention relates to a method of treating functional disability and / or pain associated with joints, tendons or connective tissue diseases, disorders or injuries comprising oral administration of a composition comprising heterologous platelet-rich plasma. The invention also relates to pharmaceutical compositions and nutritional compositions comprising heterologous platelet-rich plasma and uses thereof.
Owner:OPKO LAB EURO SL
Traditional Chinese medicine preparation for treating connective tissue disease related interstitial lung disease
ActiveCN108324833AEffective treatmentMedication is simpleAnthropod material medical ingredientsRespiratory disorderInterstitial lung diseaseConnective tissue
The invention belongs to the technical field of traditional Chinese medicines, and particularly relates to a traditional Chinese medicine preparation for treating connective tissue disease related interstitial lung disease. The traditional Chinese medicine composition is prepared from the following components in parts by weight: 20-40 parts of radix astragali, 5-20 parts of rhizoma anemarrhenae, 5-20 parts of radix bupleuri, 5-20 parts of rhizoma cimicifugae, 5-15 parts of radix platycodi, 1-10 parts of fructus schisandrae, 10-25 parts of radix salviae miltiorrhizae, 10-20 parts of radix angelicae sinensis and 3-9 parts of radix glycyrrhizae, and further contains 10-20 parts of honeycombs. The traditional Chinese medicine composition can effectively treat the connective tissue disease related interstitial lung disease, and can effectively relieve pulmonary fibrosis.
Owner:THE AFFILIATED HOSPITAL OF SHANDONG UNIV OF TCM
1,3,4-oxadiazole amide derivative compound as histone deacetylase 6 inhibitor, and pharmaceutical composition containing same
Owner:CHONG KUN DANG PHARMA CORP
Antigen-immobilized matrix membrane, kit comprising the same for detecting antinuclear antibody spectrum related to autoimmune diseases and purpose thereof
PendingCN110346576AStrong specificityIncreased sensitivityDisease diagnosisBiological testingDiseaseAntigen testing
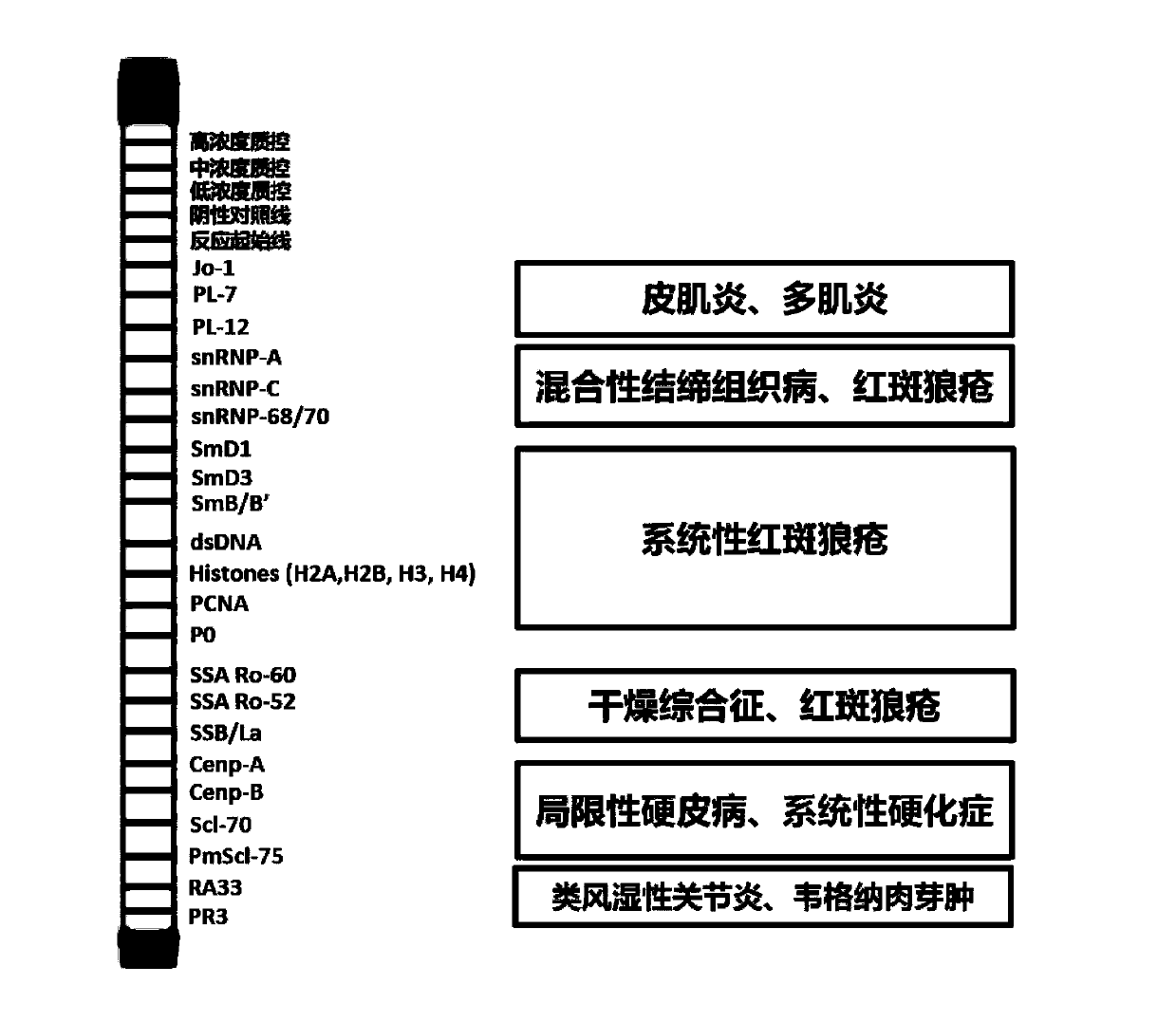
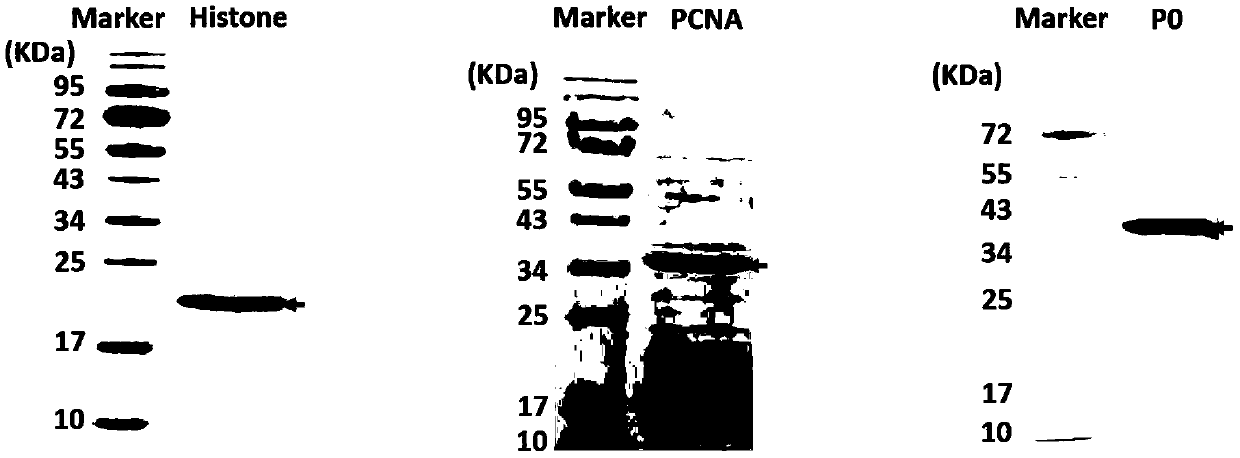
The invention discloses an antigen-immobilized matrix membrane, a kit comprising the same for detecting an antinuclear antibody spectrum related to autoimmune diseases and purpose thereof. The antigen-immobilized matrix membrane comprises but not only comprises following 22 mutually independent antigen detection lines including double-stranded DNA, histone, ribosome P0 protein, PCNA, SmD1, SmD3, SmB / B', snRNP-A, snRNP-C, snRNP68 / 70, SSA / Ro60, SSA / Ro52, SSB / La, Jo-1, PL-7, PL-12, PmScl, Scl-70, CENP-A, CENP-B, RA33 and PR3. When the kit containing the antigen-immobilized matrix membrane is usedfor detection, 22 kinds of autoimmune antibodies can be detected simultaneously; and thus diagnosis of eight kinds of common diffuse connective tissue diseases is assisted.
Owner:英诺诊断有限公司
Use of nitroxides in treating skin disease
Methods of treating inflammatory skin diseases such as rosacea, atopic dermatitis, contact dermatitis, drug eruptions, psoriasis, seborrheic dermatitis, connective tissue diseases, autoimmune disorders, urticaria or hives, and inflammation associated with skin infections by topical or systemic administration of a nitroxide containing compound are provided.
Owner:BERNSTEIN ERIC F
Connective tissue disease-associated early interstitial lung disease diagnostic kit
InactiveCN103698508AEasy to operatePeripheral blood detection method is accurateDisease diagnosisInterstitial lung diseaseSerum ige
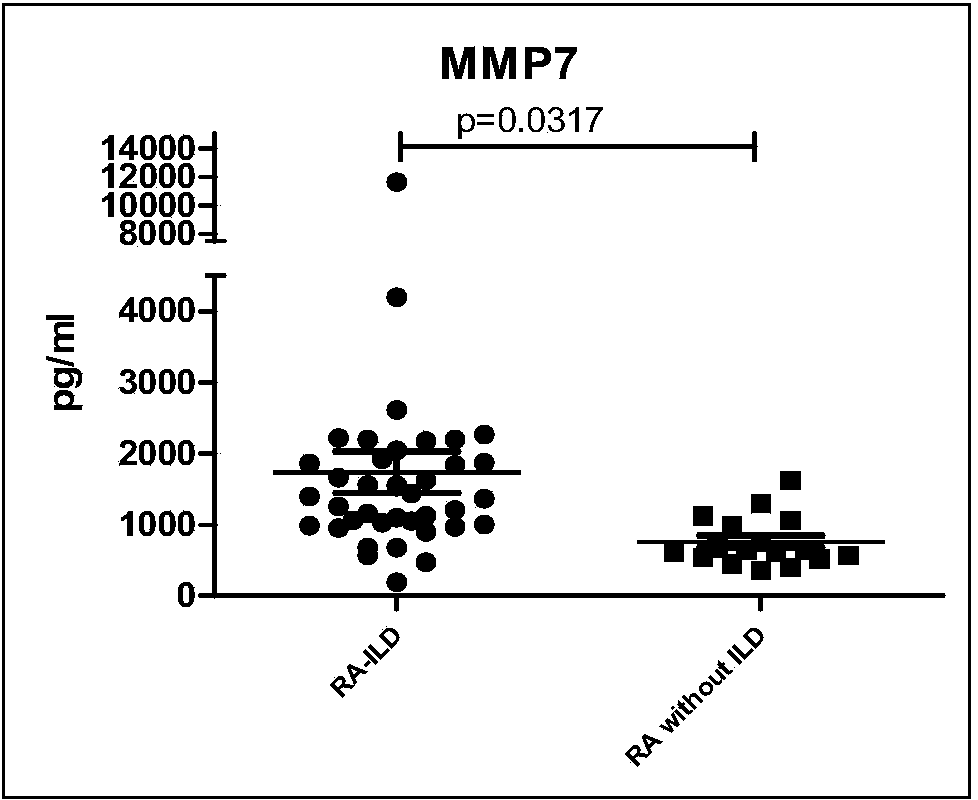
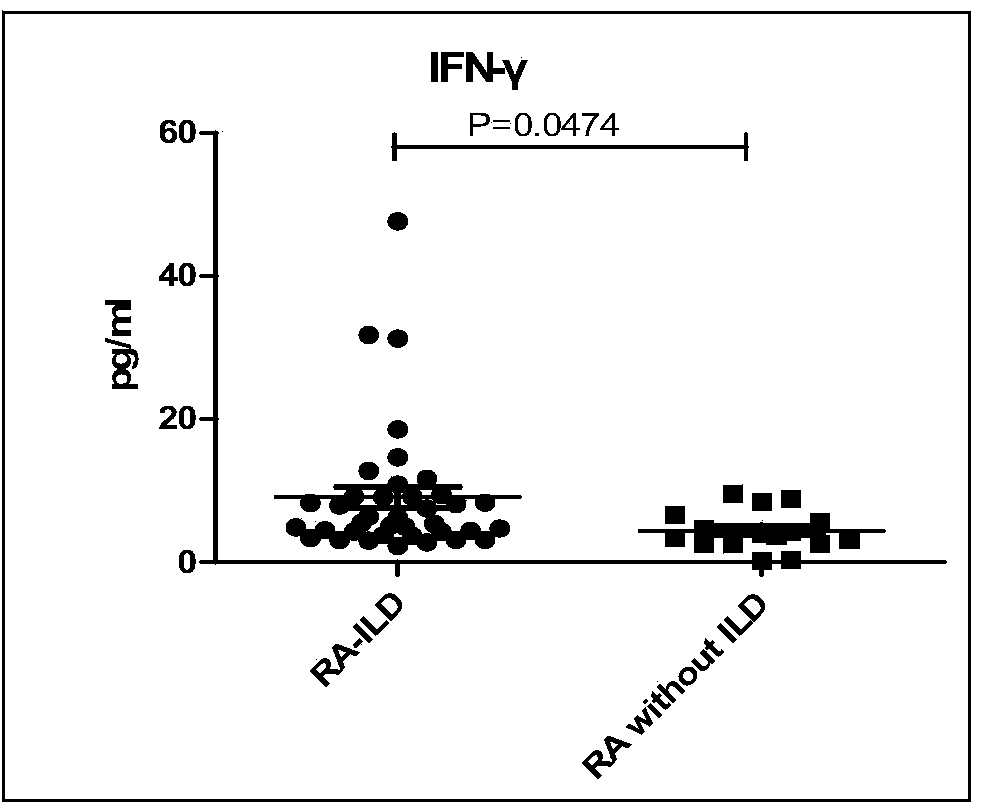
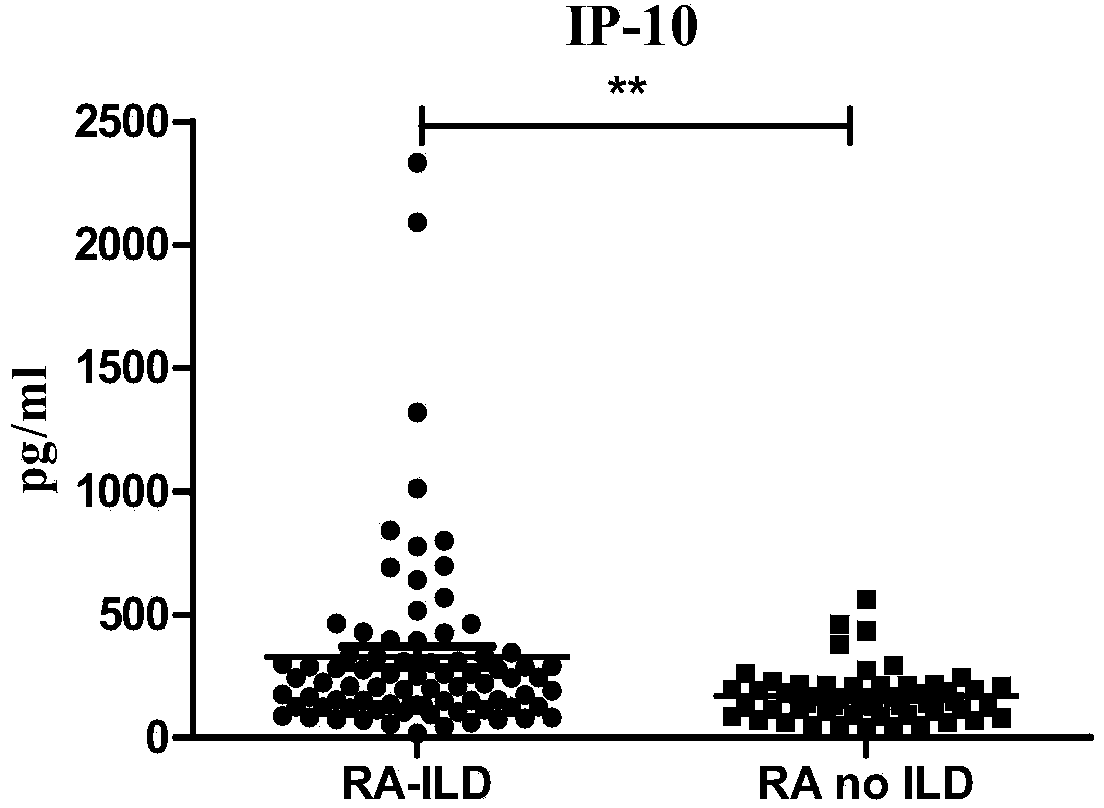
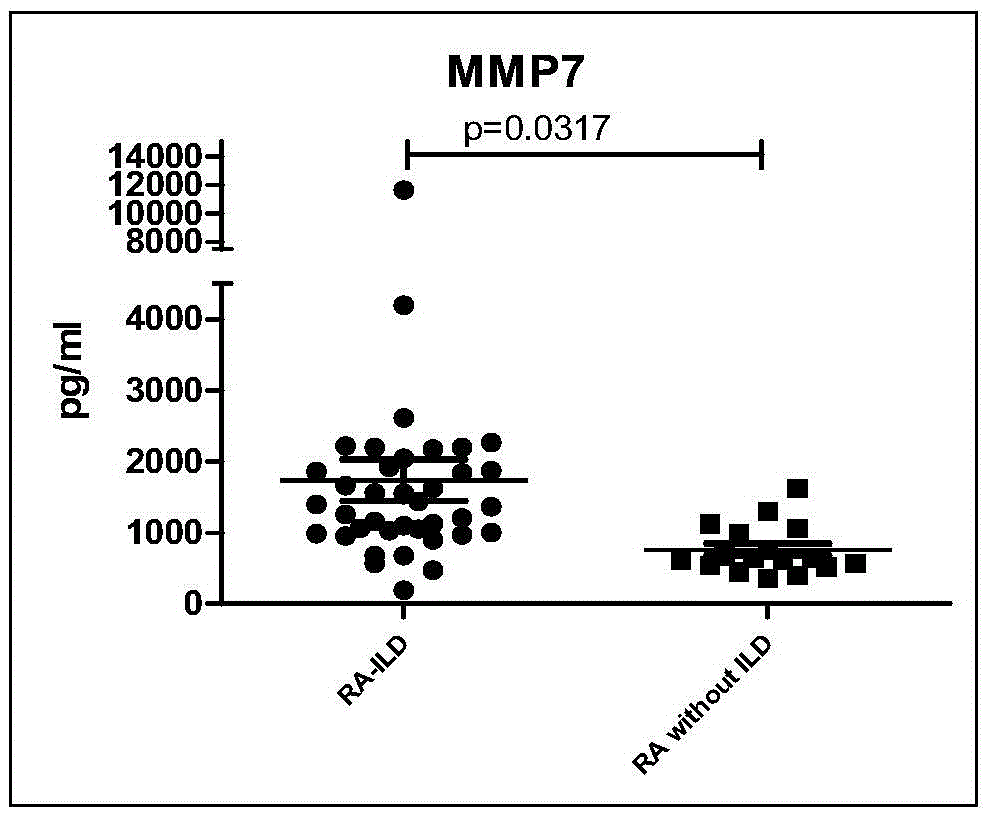
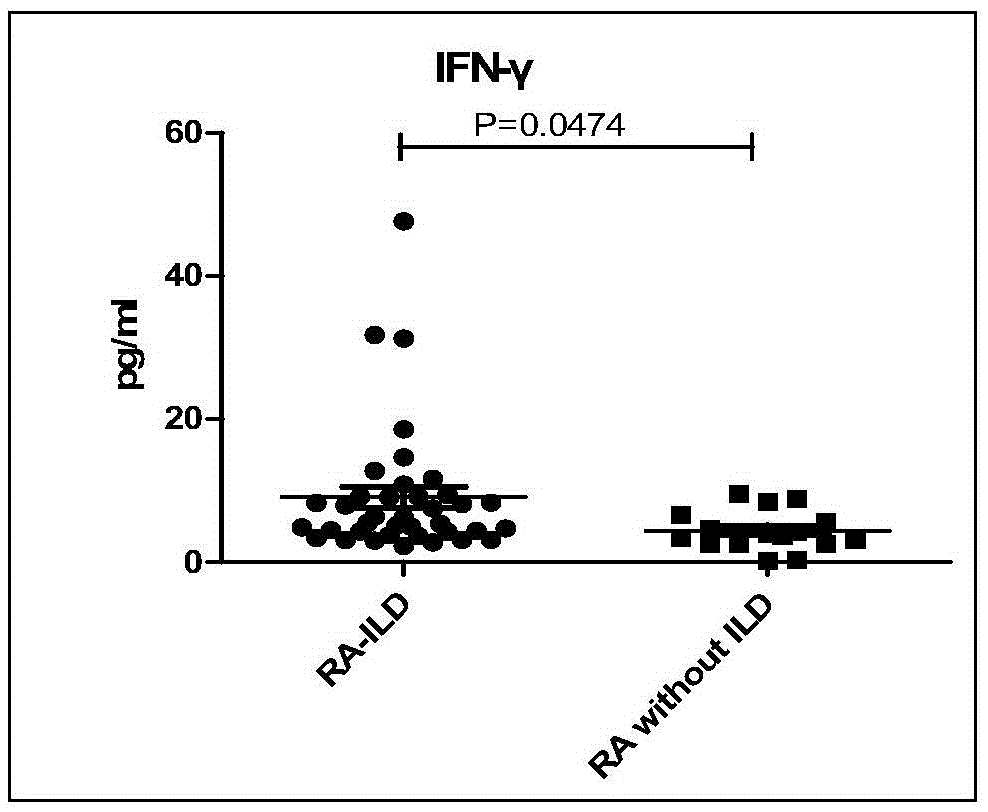
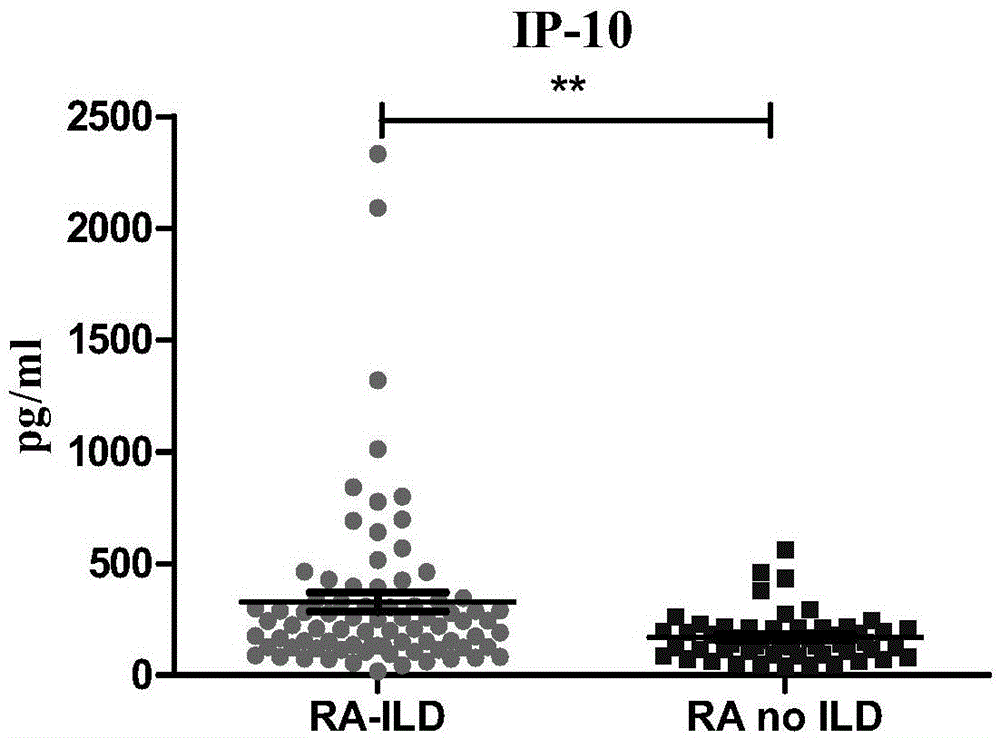
The invention discloses a connective tissue disease-associated early interstitial lung disease diagnostic kit, comprising a detection module for detecting human peripheral blood MMP7, IFN-gamma and IP-10. The commercial kit provided by the invention is sensitive, safe, reliable and easy to operate; the kit is capable of quantificationally measuring the level of specific protein cytokines and chemotactic factors in human serum, and is helpful for early diagnosis of the connective tissue disease-associated interstitial lung disease.
Owner:陈娟
Application of anti-human CD4 and anti-human CD184 monoclonal antibodies serving as markers
The invention discloses application of an compound of an anti-human CD4 monoclonal antibody and an anti-human CD184 monoclonal antibody serving as markers, in particular relates to application of the anti-human CD4 monoclonal antibody and the anti-human CD184 monoclonal antibody serving as markers for diagnosing connective tissue disease correlated interstitial pneumonia, application of the anti-human CD4 monoclonal antibody and the anti-human CD184 monoclonal antibody to preparation of a reagent or kit for diagnosing the connective tissue disease correlated interstitial pneumonia, or application of the anti-human CD4 monoclonal antibody and the anti-human CD184 monoclonal antibody to preparation of a reagent or kit for judging the severity degree, regression or prognosis of the connective tissue disease correlated interstitial pneumonia. The invention further provides a corresponding kit.
Owner:SHANGHAI CITY JIADING DISTRICT CENT HOSPITAL
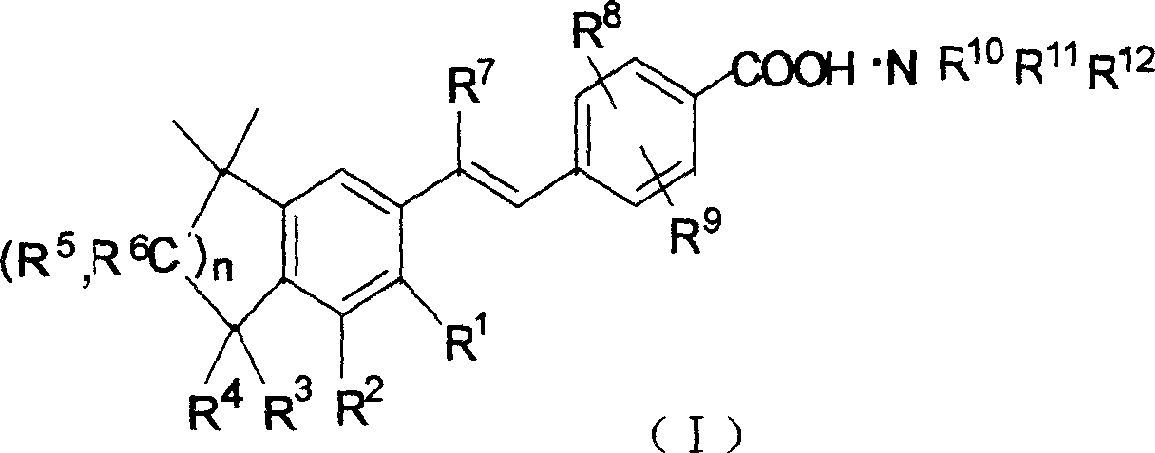
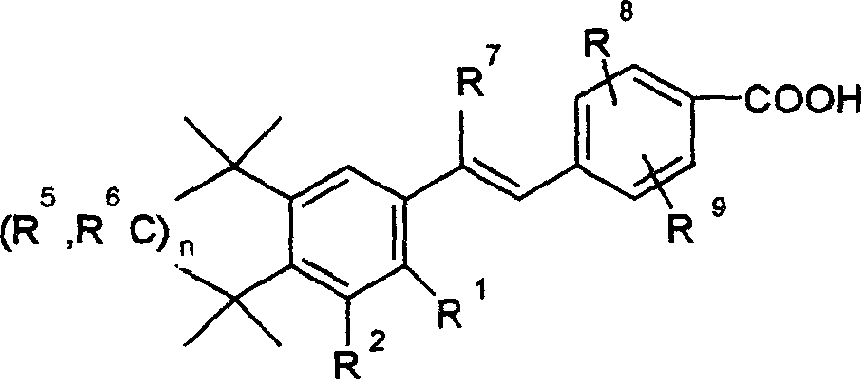
Stibene derivative organic amine salt, its preparation method, use and medicinal composition
The invention relates to an organic amine salt of a stilbene derivative, a preparation method, an application and a pharmaceutical composition thereof. The present invention provides various organic amine salts of stilbene derivatives. Its preparation method is to react stilbene derivatives with ammonia or organic amine compounds to generate corresponding organic amine salts. The compound of the present invention can be used to prepare medicines for preventing and treating tumors and precancerous lesions, and medicines for treating connective tissue diseases or skin diseases; it can be prepared into various oral, injection and external preparations. The water solubility of the compound of the present invention is greatly increased compared with its parent compound stilbene derivative, so the dosage is small and the curative effect is good.
Owner:CHONGQING HUAPONT PHARMA
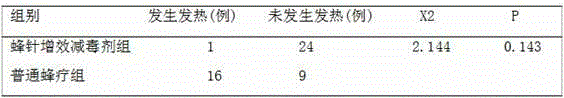
Bee needle attenuation and effect-increase oral liquid and preparation method thereof
The present invention provides a bee needle attenuation and effect-increase oral liquid and a preparation method thereof. The bee needle attenuation and effect-increase oral liquid comprises a cortex mori and clematis chinensis water extract and honey according to a volume ratio of (0.5-1.5):1, wherein cortex mori and clematis chinensis are taken according to a mass ratio of (2-5):(3-6) and then are subjected to mixing and water boiling extraction to obtain the cortex mori and clematis chinensis water extract. According to the present invention, the bee needle attenuation and effect-increase oral liquid is matched with the bee needle therapy so as to effectively reduce adverse reactions of patients after the bee needle therapy, enhance the bee needle therapy effect, provide the new assisted therapy method for rheumatoid arthritis, ankylosing spondylitis, other connective tissue diseases and rheumatic diseases, substantially increase the bee needle therapy safety, and provide the new idea and the effective method for overcome of the biggest limiting factor of the promotion of the bee needle therapy.
Owner:昆明市中医医院
Method of treating pulmonary disease with interferons
A method of treating a pulmonary disease such as, for instance idiophathic pulmonary fibrosis (IPF), mixed connective tissue disease and asthma, comprising administering an aerosolized interferon such as interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) alone or in combination with other therapeutic agents are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Treatment of connective tissue diseases of the skin
ActiveUS8426475B2Good treatment effectImprove lesionBiocideHydroxy compound active ingredientsSkin sensitizationDisease
The present invention provides effective and safe medicaments for the treatment of connective tissue diseases of the skin, particularly with respect to the treatment of cutaneous forms of Lupus Erythematous. The medicaments comprise as the therapeutically active ingredient a beta2 adrenoceptor agonist. The invention furthermore relates to dermatological compositions without skin sensitization properties and which contain enantiomerically pure or enriched R-enantiomers of a beta2 adrenoceptor agonist.
Owner:ASTION PHARMA AS
A diagnostic kit for early pulmonary interstitial lesions of connective tissue diseases
InactiveCN103698508BEasy to operatePeripheral blood detection method is accurateDisease diagnosisInterstitial lung diseaseCytokine
The invention discloses a connective tissue disease-associated early interstitial lung disease diagnostic kit, comprising a detection module for detecting human peripheral blood MMP7, IFN-gamma and IP-10. The commercial kit provided by the invention is sensitive, safe, reliable and easy to operate; the kit is capable of quantificationally measuring the level of specific protein cytokines and chemotactic factors in human serum, and is helpful for early diagnosis of the connective tissue disease-associated interstitial lung disease.
Owner:陈娟
Plant extract composite buccal tablet
PendingCN109602820AImprove immunityGood treatment effectHeavy metal active ingredientsNervous disorderDegenerative DisorderChronic degenerative disease
The invention discloses a plant extract composite buccal tablet, comprising the following raw materials: L-leucine, resveratrol, quercetin, anthocyanin, fisetin, pterostilbene, nicotinic acid, folic acid, chromium, magnesium, Coenzyme Q10, curcumin, lipoic acid, green tea extract, and milk thistle extract. According to the invention, the purely natural plant substances such as green tea extract and milk thistle extract added to the buccal tablet have significant therapeutic effect on respiratory diseases, digestive diseases, circulatory diseases, urinary system diseases, endocrine diseases, metabolic diseases, lymphatic diseases, neurological system diseases, connective tissue disease, gynecological diseases, otolaryngology diseases, stomatological diseases, orthopedic diseases, dermatological diseases, oncological diseases and other chronic degenerative diseases. Meanwhile, the buccal tablet is low in cost and free of side effects, has the efficacy of clearing heat and detoxifying, moving qi and activating blood, nourishing and strengthening body, regulating qi and invigorating stomach, benefiting qi and tonifying kidney, tranquilizing and tonifying spleen, and also is capable ofenhancing physical fitness to improve the immunity of the body to diseases.
Owner:烟台恩美诺生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com