A diagnostic kit for early pulmonary interstitial lesions of connective tissue diseases
A technology for pulmonary interstitial lesions and diagnostic kits, which is applied in disease diagnosis, measuring devices, instruments, etc., can solve problems such as difficulty in repeating, delaying treatment timing, expensive detection methods, etc., and achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
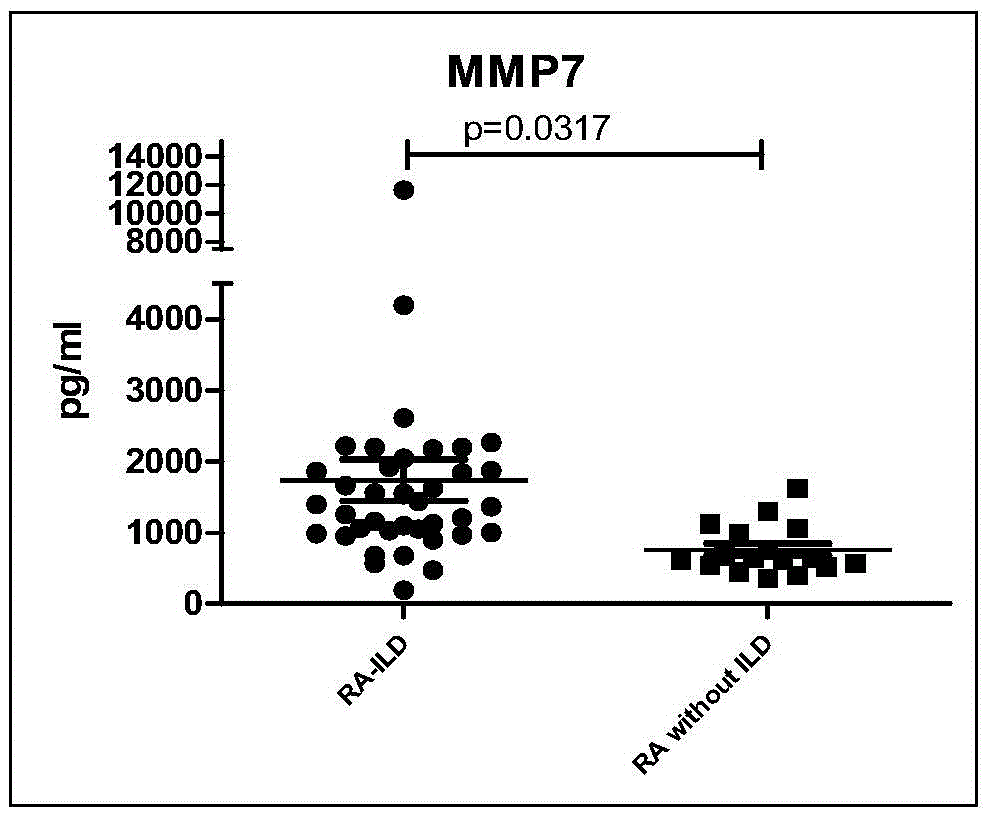
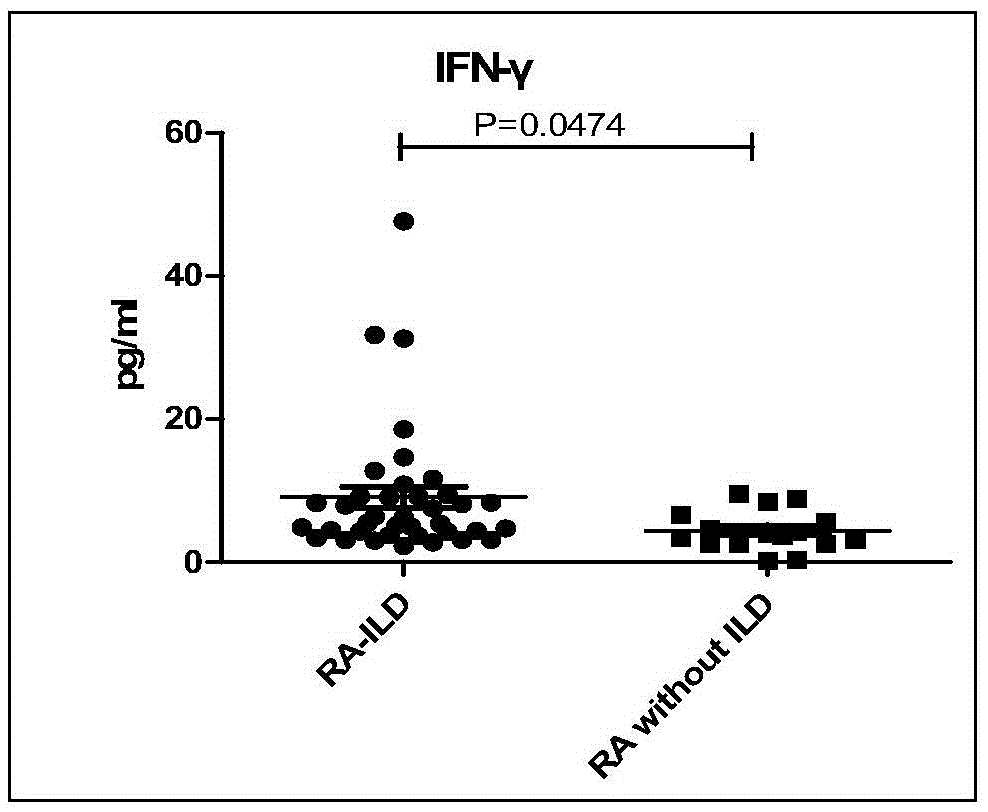
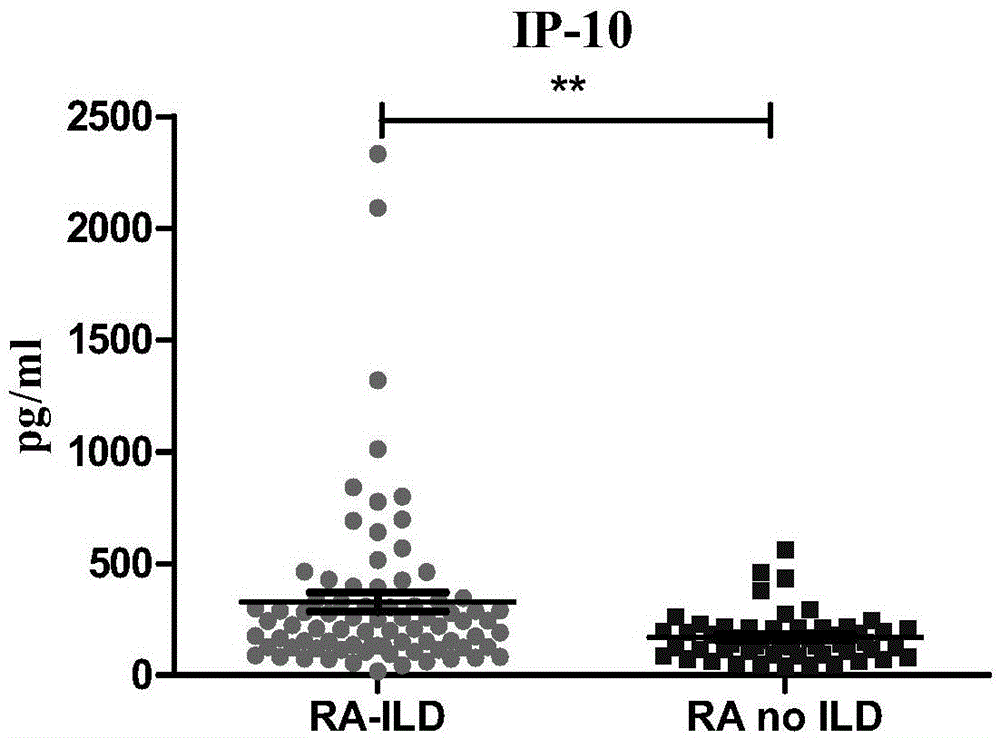
[0032] 135 cases of rheumatoid arthritis (RA) patients were tested in actual cases, and it was confirmed that the peripheral serum matrix metalloproteinase 7 (MMP7), interferon-γ (IFN-γ) and chemotaxis in patients with connective tissue disease pulmonary interstitial lesions Increased levels of factor IP-10. Detecting the levels of matrix metalloproteinase 7 (MMP7), interferon-γ (IFN-γ) and chemokine IP-10 in peripheral serum of patients can diagnose early pulmonary interstitial lesions of connective tissue disease. The specific verification process is as follows:
[0033] A total of 135 patients with rheumatoid arthritis (RA) were recruited from the Department of Rheumatology and Immunology, The First Affiliated Hospital of Xiamen University. All met the diagnostic criteria for RA in 1982 by the American College of Rheumatology. All the enrolled patients underwent high-resolution pulmonary CT (HRCT) and pulmonary function (PFTs) examinations, and statistics of clinical join...
Embodiment 2
[0046] A diagnostic kit for early-stage pulmonary interstitial lesions of connective tissue disease prepared according to the experimental results of the above embodiment 1, comprising a first ELISA detection component for detecting MMP7 in peripheral serum of patients with connective tissue disease, and a second ELISA for detecting IFN-γ Assay kit and third ELISA assay kit to detect IP-10.
[0047] The preparation method of the above-mentioned 96-well detection plate is as follows:
[0048] Coat 200ng / mL antibody on a 96-well plate at 100uL per well, the antibody diluent is PBS, incubate the 96-well plate at 37°C for 2h; incubate for two hours, wash the plate once, add 200uL of blocking solution to each well, Block at 37°C for 2 hours, wash the plate once after blocking, and place the plate in a drying room to dry before use; the components of the blocking solution are: 1XPBS + 0.5% casein + 2% gelatin + 0.1% preservative (proclin-300).
[0049] The detection process of each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com