Kit for detecting anti-moesin antibody
A detection kit, membrane protein technology, applied in the biological field to achieve the effect of high sensitivity, specificity, and high-throughput detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
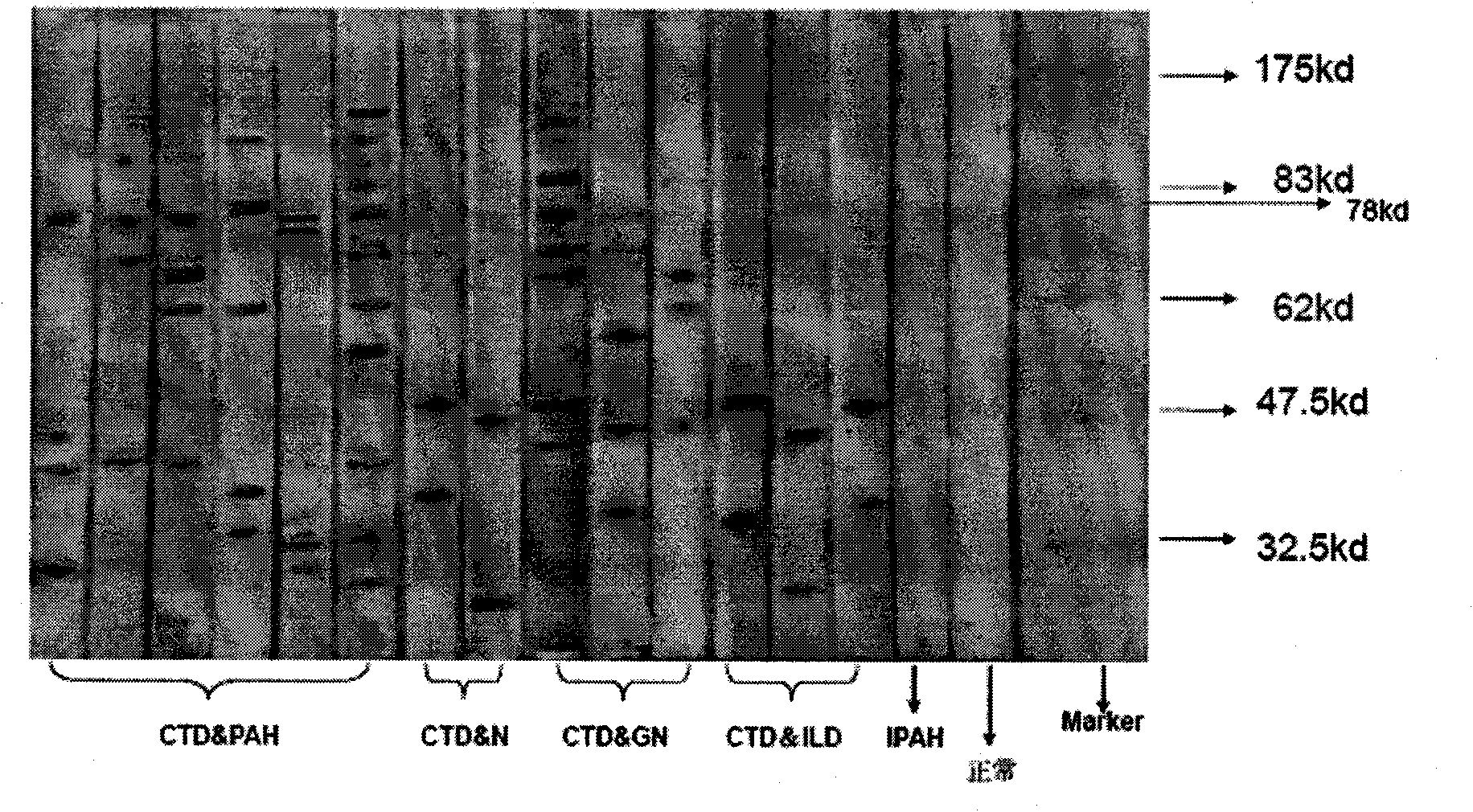
[0068] Example 1 Determination of the AECA main target antigen moesin of CTD-related PAH
[0069] Step 1: AECA detection in patients with CTD-associated PAH
[0070] The positive rate of anti-endothelial cell antibody in patients with CTD-associated PAH was detected by cell-ELISA and Western blotting, and the relationship between AECA and CTD-associated PAH specificity and disease clinical activity indicators was analyzed.
[0071] 1. Research object and method
[0072] 1.1 Research object
[0073] The research subjects were patients with connective tissue disease-associated pulmonary arterial hypertension who were diagnosed and treated in the Department of Rheumatology and Immunology of Peking Union Medical College Hospital from January 2006 to May 2007. The experimental group selected 68 patients with CTD-associated PAH (CTD-PAH) (only with pulmonary arterial 12 patients with idiopathic pulmonary arterial hypertension (IPAH) and 61 patients with CTD without PAH in the cont...
Embodiment 2
[0127] Embodiment 2 moesin protein gene cloning, prokaryotic expression and purification
[0128] Step 1: Gene Recombination of Moesin
[0129] 1.1 Acquisition of recombinant protein moesin cloning template: RNA was extracted from gastric cancer tissue and reverse transcribed into cDNA.
[0130] 1.2 The full-length cDNA sequence of moesin protein provided by genebank is as follows,
[0131] 1.2.1 Nucleotide sequence: (for the sequence see >gi|53729335:199-1932 Homo sapiens moesin (MSN), mRNA 1734bp).
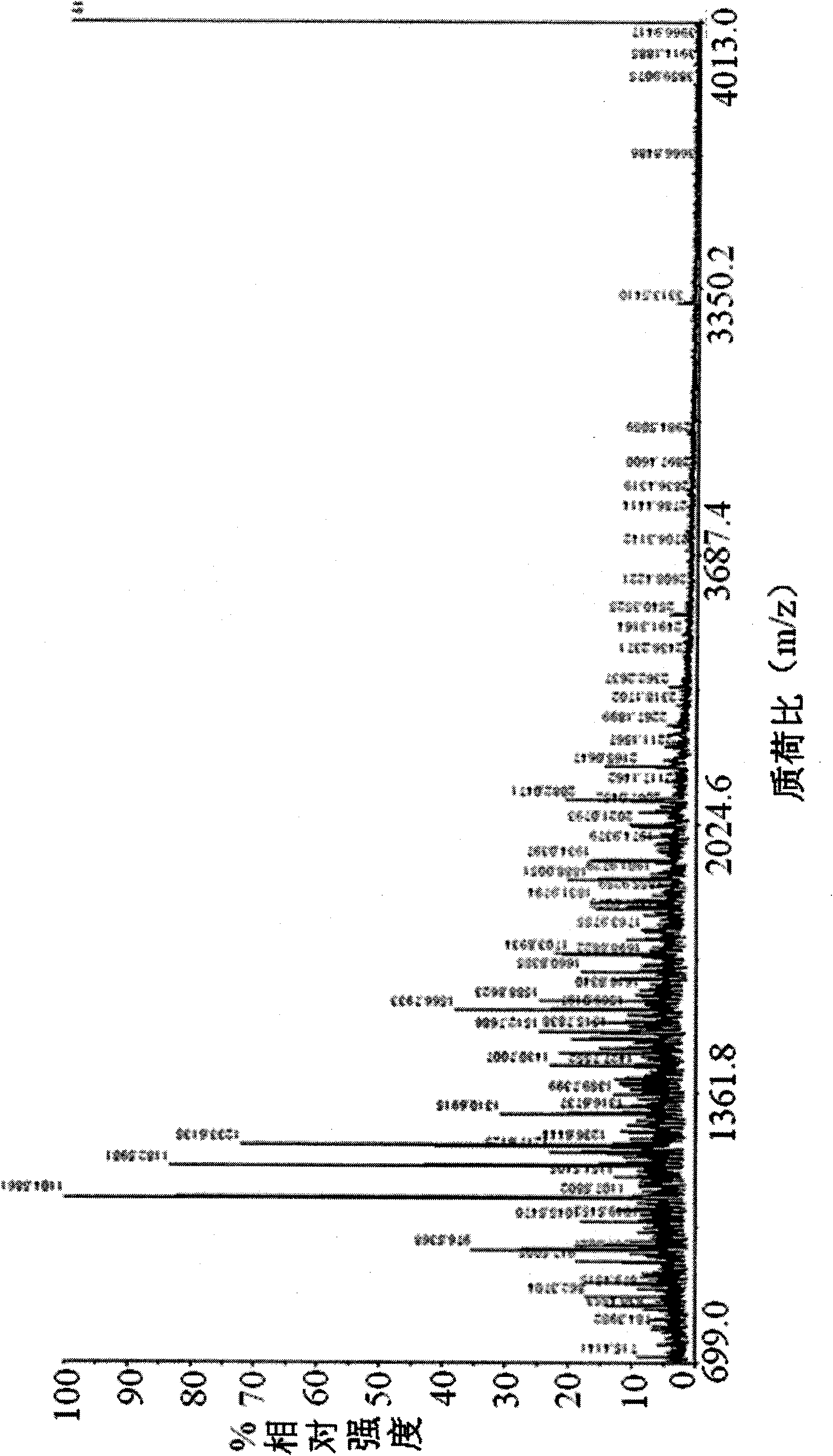
[0132] 1.2.2 Protein sequence: see SEQ ID NO: 1 and image 3 . image 3 In , the amino acid composition of Moesin and the matching amino acid sequence by mass spectrometry (the matching amino acid sequence is marked in bold and underlined).
[0133] 1.2.3 Moesin protein amplification primer sequences were designed as follows:
[0134] The sequence corresponding to Moesin-top (EcoR I) is SEQ ID NO: 2
[0135] 5'-CCGGAATTCATGCCCAAAACGATCAGT-3'
[0136] The corresponding seq...
Embodiment 3
[0152] The preparation of embodiment 3 anti-moesin antibody detection kit
[0153] The first step: exploration experiment of optimal reaction conditions
[0154] 1.1 Antigenicity evaluation and selection basis of two recombinant proteins
[0155] The antigenicity of the two recombinant protein antigens was evaluated by the indirect enzyme-linked immunosorbent method. The specific method was: the antigen was diluted to 1 μg / ml, 2 μg / ml, 4 μg / ml, 5 μg / ml, 10 μg / ml and then coated with enzyme labeling Plate, clinical collection of anti-moesin body test results are strong positive, weakly positive and negative serum samples 1 each. Within the concentration range of the above-mentioned antigen coating, the selection of antigen 1# can clearly distinguish three different serum samples, and the difference is significant. Therefore, antigen 1# is used as the antigen protein for detection, which is considered to have strong antigenicity (sensitivity) and lower background values (spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com