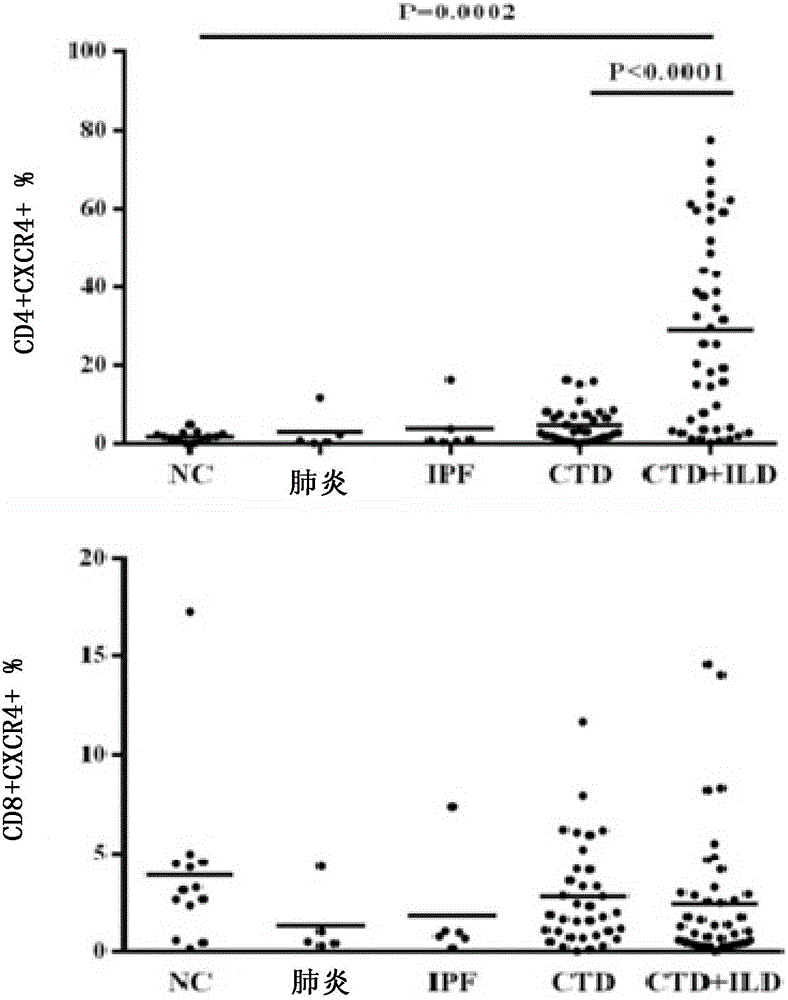
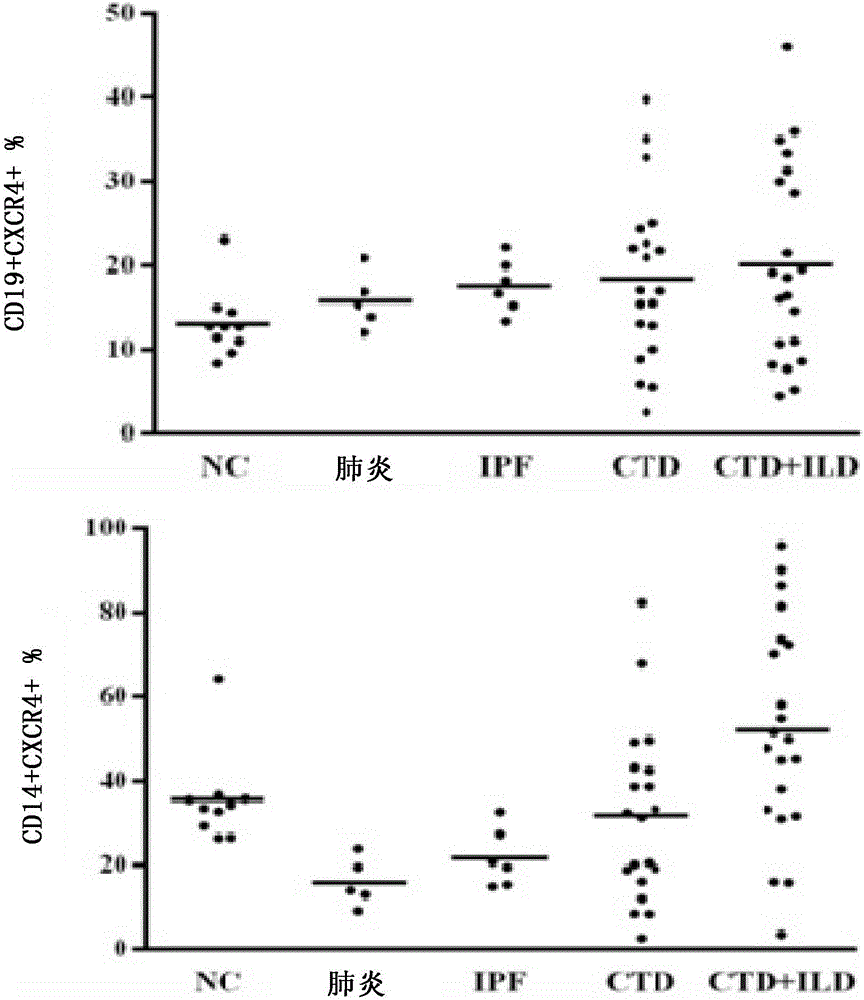
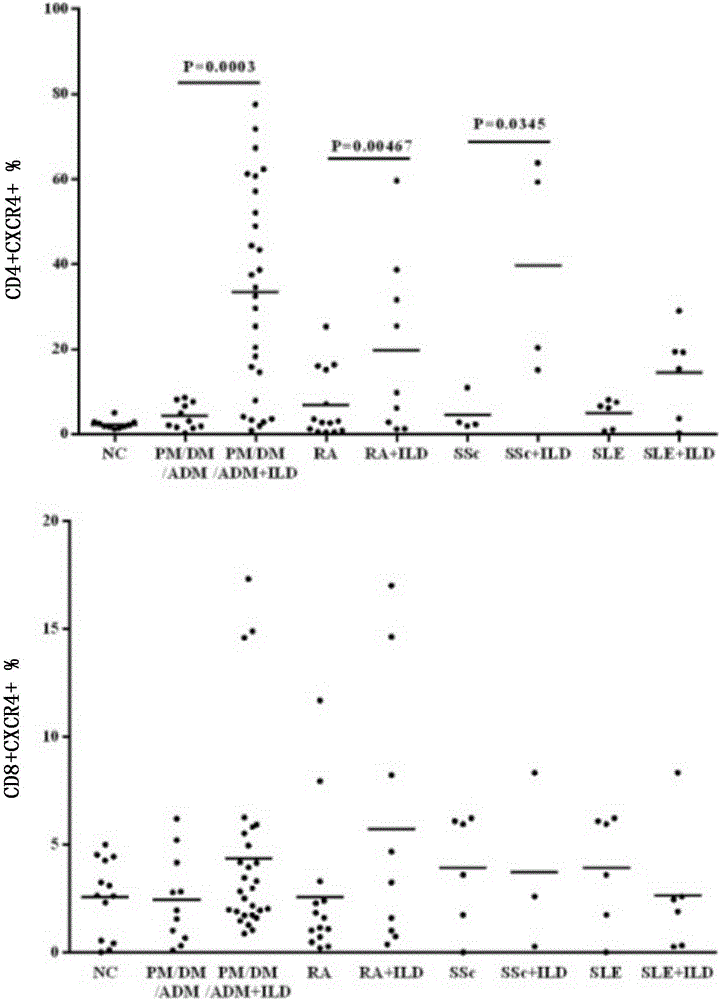
Application of anti-human CD4 and anti-human CD184 monoclonal antibodies serving as markers
A technology of monoclonal antibody and application, which is applied in the medical field and can solve problems such as high positive rate, no specific diagnostic value of CTD-ILD, unstable ELISA detection results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Isolation of PBMC from peripheral blood of patients
[0073] Take 5ml of heparin anticoagulant blood from the patient, mix it upside down repeatedly; dilute the blood sample with an equal amount of PBS.
[0074] Inject the diluted blood sample obliquely and slowly along the wall above 4ml of lymphatic separation solution; centrifuge at 1200g for 10min in a slow-rising and slow-falling manner.
[0075] Absorb part of the cells in the turbid zone in the centrifuge tube, transfer to another centrifuge tube added with PBS, 600g, and centrifuge for 7 minutes quickly;
[0076] Discard the supernatant, resuspend in 200 μl, count the cells, and dilute to a lymphocyte count of 0.5×10 9 / g, standby.
Embodiment 2
[0078] Preparation of mixed fluorescent antibodies
[0079] Adjust the concentration of anti-human CD4 monoclonal antibody (Abcam Company) to 15 mg / ml and anti-human CD184 monoclonal antibody (Abcam Company) to 25 mg / ml respectively, and place them in an ice bath for magnetic stirring for 10 min, without foaming during the stirring process.
[0080] FITC (Sigma Company) was weighed according to 0.01 mg / mg protein for labeling CD184.
[0081] APC (Sigma Company) was weighed according to 0.05 mg / mg protein for labeling CD4.
[0082] Slowly add fluorescein to the antibody protein solution under stirring, this step is completed within 5 minutes, place the mixed solution at 4 degrees, and continue magnetic stirring for 18 hours.
[0083]The protein mixture was taken out, centrifuged at 2500 rpm / min for 25 min, and dialyzed overnight at 4°C using buffered saline with a pH value of 8.0.
[0084] The protein mixture dialyzed overnight was passed through a glucose gel G50 column to r...
Embodiment 3
[0088] Double-antibody flow cytometry combined detection method based on the proportion of CD4+CD184+T cells in peripheral blood PBMC
[0089] Take 5ml of fresh heparin anticoagulated blood from the patient, and mix it upside down repeatedly; extract PBMC from the patient's peripheral blood.
[0090] Count the extracted PBMC with a hematology analyzer, and dilute the PBMC to 0.5×10 based on the number of lymphocytes. 9 / g.
[0091] Aspirate 100 μl of diluted PBMC, add 10 μl of mixed antibody prepared in Example 2, and incubate in the dark for 15 minutes.
[0092] Add 200μl PBS, mix well, and perform flow cytometry detection on the machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com