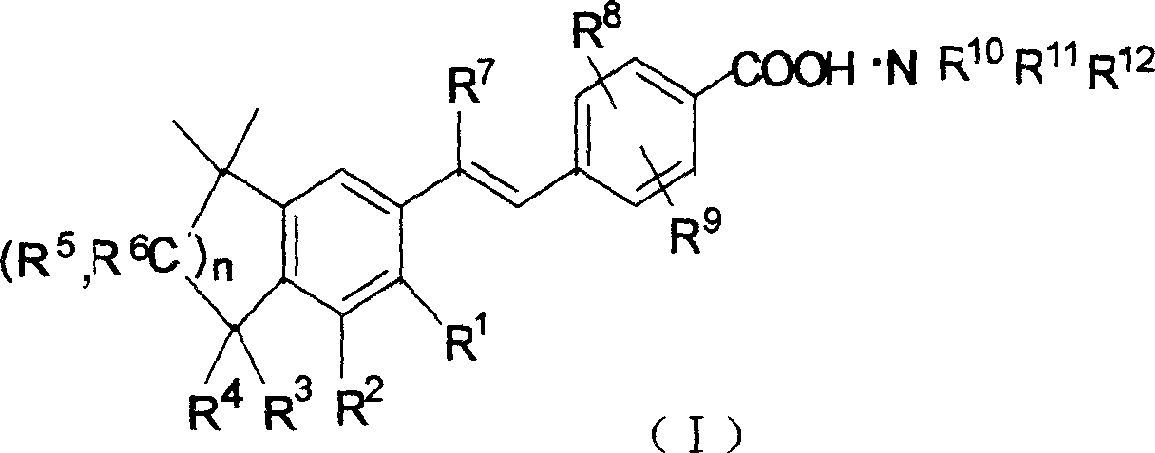
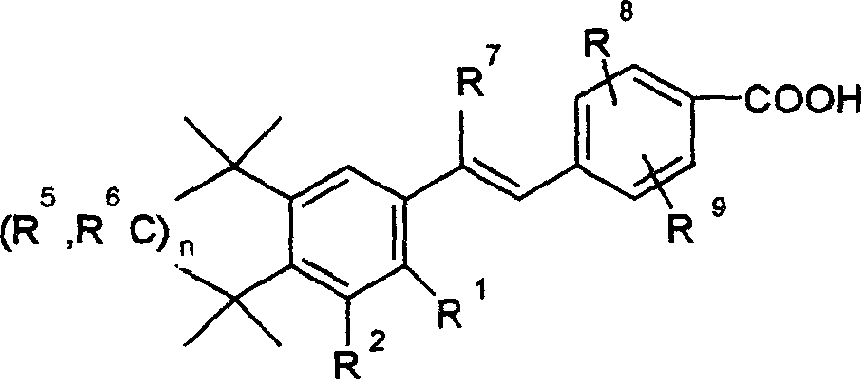
Stibene derivative organic amine salt, its preparation method, use and medicinal composition
A technology of organic amine salts and organic amines, which is applied in the field of stilbene derivative organic amine salts and their preparation, use and pharmaceutical composition, and can solve the problems of low water solubility of stilbene derivatives, pharmacological action intensity and range limitations, etc. problems, to achieve good curative effect, wide range of pharmacological effects, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
[0048] The preparation of formula I compound in embodiment 1~7
[0049] The compound of formula I is prepared from the compound of formula II and the compound of formula III through neutralization reaction.
[0050] The compound of formula II used is 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoic acid, which The structural formula is:
[0051]
[0052] Formula II
[0053] The compound of formula III used is shown in the following table
[0054] Compound of formula III
[0055]
Embodiment 1
[0056] Example 1 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoic acid 2-hydroxymethyl yl-2-amino-1,3-propanediol salt
[0057] Add 3.5g (10mmol) 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propene in a 250ml three-necked flask Base] benzoic acid, 130ml methanol, 20ml chloroform, heat up and reflux, add 1.21g (10mmol) trimethylolmethylamine after dissolution is complete, insoluble matter is precipitated, heat up and reflux for 2 hours, freeze for 30 minutes after cooling, suction filter, freeze Washed with methanol, recrystallized and dried to obtain 4.4 g of the title compound as a white solid. The yield is 93.8%, and the melting point is 190.5-192.6°C.
[0058] 13 CNMR(DMSO):170.196、144.335、143.774、140.561、139.975、137.826、129.262、128.525、126.464、126.364、123.723、123.414、60.955、60.054、34.942、34.747、34.104、33.865、31.785、31.697、17.504。
[0059] C(%)
Embodiment 2
[0060] Example 2 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoic acid meglumine salt
[0061] Dissolve 2g of 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoic acid in 110ml of chloroform Dissolve, add 1.15 g of meglumine in 20 ml of DMF dropwise under reflux, and reflux for 1.5 hours after addition, evaporate the oily substance of the solvent to dryness under reduced pressure, add 10 ml of absolute ethanol, dissolve it, and precipitate the solid in the freezer.
[0062] The title compound was obtained as a white solid by suction filtration, and dried to obtain 2.6 g, melting point 212-214°C, yield 85.8%.
[0063] 13 CNMR(DMSO):170.817、144.418、143.836、140.665、138.749、137.765、135.826、129.336、128.545、126.572、126.510、123.802、123.497、71.638、70.954、70.615、69.010、63.713、51.769、35.003、74.810、34.194、33.962 , 33.476, 31.865, 31.786.17.598.
[0064] C(%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com