A kind of pharmaceutical composition for treating multiple sclerosis
A multiple sclerosis and composition technology, applied in the field of biomedicine, can solve the problems of not being able to reduce the number of plasma cells and self-reactive memory B cells, repeated and exacerbated MS conditions, etc., to reduce the level, reduce the reaction of autoantigens, The effect of reducing the number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
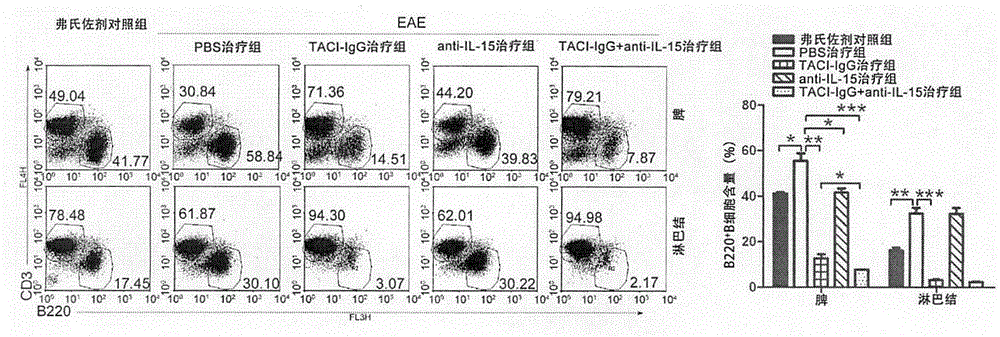
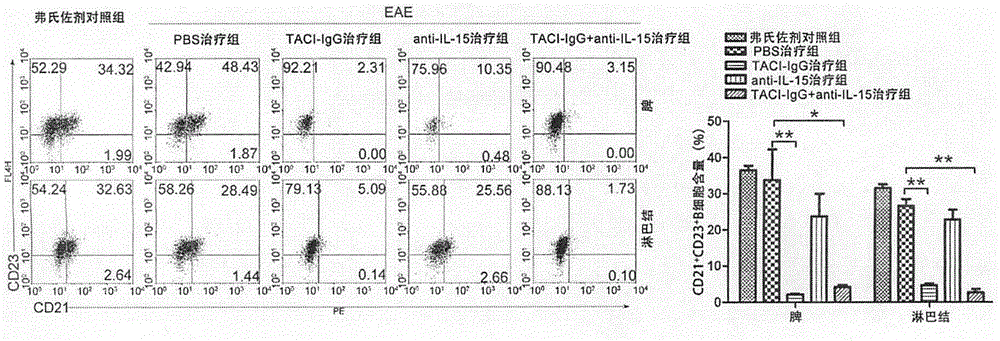
[0031] Example 1 Effect of TACI-IgG and Anti-IL-15 Antibody Combined Therapy on B Cells
[0032] 1. Materials and methods
[0033] 1. Materials
[0034] BALB / c mice were purchased from the Animal Center of the Academy of Military Medical Sciences; mouse myelin oligodendrocyte glycoprotein 33-55 (MOG33-55), complete Freund's adjuvant (CFA), and pertussis toxin were purchased from Sigma; rabbits Anti-mouse B220 monoclonal antibody, rabbit anti-mouse CD3 monoclonal antibody, rabbit anti-mouse CD21 monoclonal antibody, rabbit anti-mouse CD23 monoclonal antibody were purchased from Abcam; BAFF inhibitor TACI-IgG, anti-mouse IL-15 neutralizing antibody were purchased from R&D company.
[0035] 2. Method
[0036] 2.1. Construction of EAE mouse model
[0037] Dilute MOG35-55 to 2mg / mL with 0.01mol / L PBS, then fully mix the diluted solution with an equal amount of complete Freund’s adjuvant, and pump the emulsion into a water-in-oil state through a three-way tube, which is to induc...
Embodiment 2
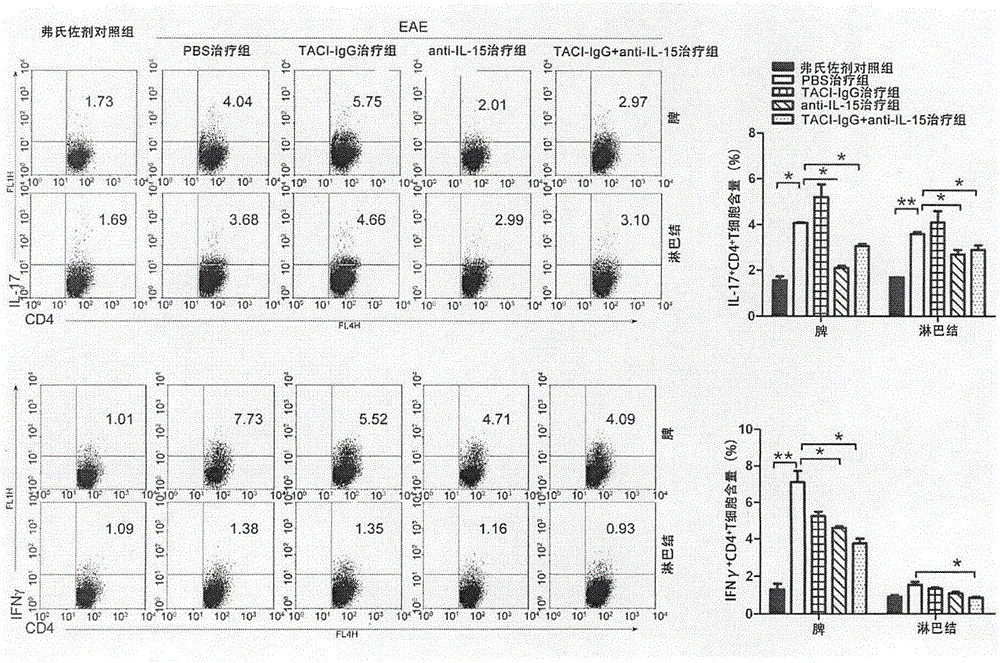
[0060] Example 2 Effect of TACI-IgG and Anti-IL-15 Antibody Combined Therapy on Helper T Cells (Th1, Th17)
[0061] 1. Materials and methods
[0062] 1. Materials
[0063] BALB / c mice were purchased from the Animal Center of the Academy of Military Medical Sciences; mouse myelin oligodendrocyte glycoprotein 33-55 (MOG33-55), complete Freund's adjuvant (CFA), and pertussis toxin were purchased from Sigma; rabbits Anti-mouse CD4 monoclonal antibody, rabbit anti-mouse IL-17 monoclonal antibody, rabbit anti-mouse IFNγ monoclonal antibody were purchased from Abcam; BAFF inhibitor TACI-IgG, anti-mouse IL-15 neutralizing antibody were purchased from R&D Company.
[0064] 2. Method
[0065] 2.1. Construction of EAE mouse model
[0066] Dilute MOG35-55 to 2mg / mL with 0.01mol / L PBS, then fully mix the diluted solution with an equal amount of complete Freund’s adjuvant, and pump the emulsion into a water-in-oil state through a three-way tube, which is to induce EAE antigenic emulsion...
Embodiment 3
[0088] Example 3 Effect of TACI-IgG and Anti-IL-15 Antibody Combined Therapy on Memory T / B Cells
[0089] 1. Materials and methods
[0090] 1. Materials
[0091] BALB / c mice were purchased from the Animal Center of the Academy of Military Medical Sciences; mouse myelin oligodendrocyte glycoprotein 33-55 (MOG33-55), complete Freund's adjuvant (CFA), and pertussis toxin were purchased from Sigma; rabbits Anti-mouse B220 monoclonal antibody, rabbit anti-mouse CD44 monoclonal antibody, rabbit anti-mouse CD27 monoclonal antibody, rabbit anti-mouse CD3 monoclonal antibody were purchased from Abcam; BAFF inhibitor TACI-IgG, anti-mouse IL-15 neutralizing antibody were purchased from R&D company.
[0092] 2. Method
[0093] 2.1. Construction of EAE mouse model
[0094] Dilute MOG35-55 to 2mg / mL with 0.01mol / L PBS, then fully mix the diluted solution with an equal amount of complete Freund’s adjuvant, and pump the emulsion into a water-in-oil state through a three-way tube, which is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com