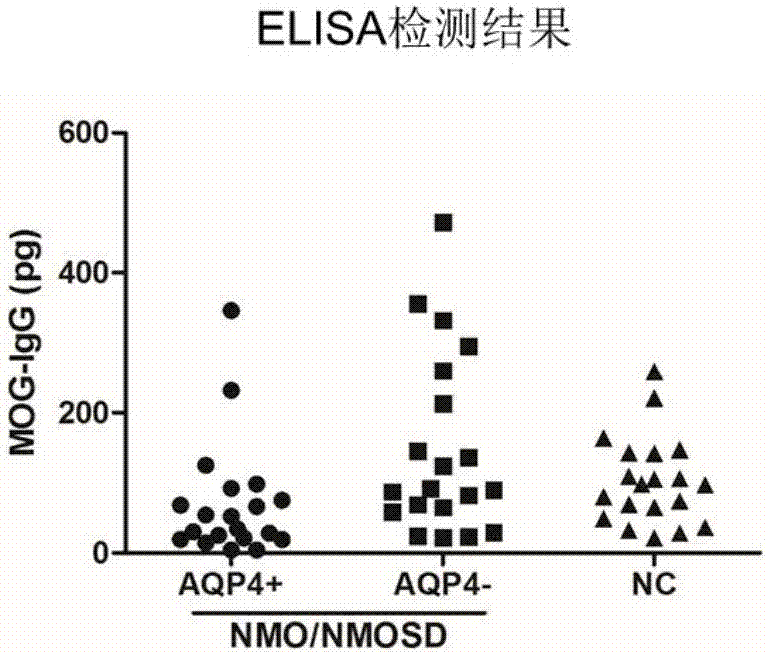
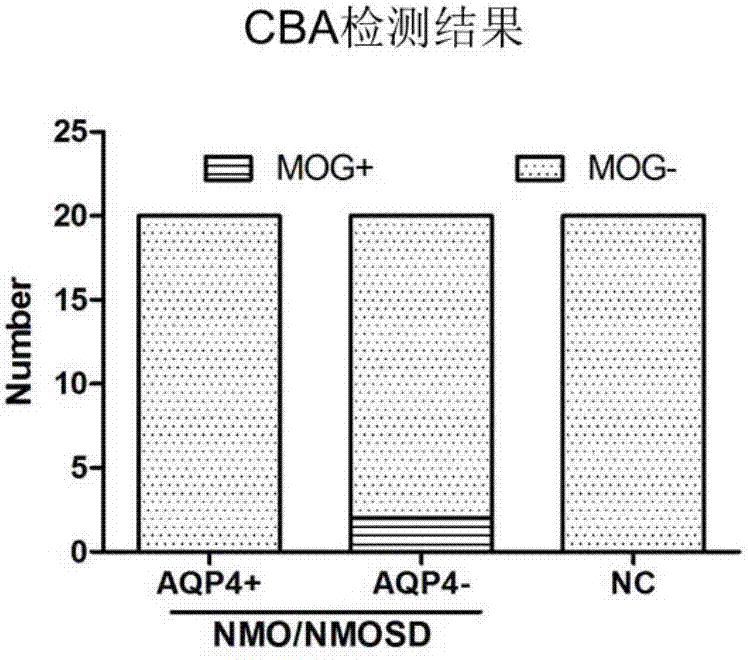
Preparation of cell growing slide assembly of CBA (cytometric bead array) kit for detecting MOG-IgG, CBA detection kit and application of CBA detection kit
A kit, the technology of MOG-293T, is applied in the direction of cells, applications, and animal cells modified by the introduction of foreign genetic material. It can solve the problems of production and sales of untested products, and achieve easy storage, convenient comparison, and improved accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of preparation method of the CBA detection kit that is used for clinical sample MOG autoimmune IgG antibody detection, described preparation method comprises the following steps:
[0045] S1: MOG plasmid construction
[0046] (1) Using human oligodendrocytes as materials, extracting total mRNA;
[0047] (2) Using a reverse transcription kit to reverse transcribe the mRNA into cDNA;
[0048] (3) Design MOG-specific PCR primers to expand the full-length MOG cDNA; the forward primer sequence of the MOG-specific PCR primers is: ATGGCAAGCTTATC AAGACCC, and the reverse primer sequence is: GAAGGGATTTCGTAGCTCTTC;
[0049] (4) Cloning the amplified MOG cDNA and connecting it to the pLVX-IRES-mcherry lentiviral plasmid vector;
[0050] (5) Then transform the pLVX-IRES-mcherry lentiviral plasmid, shake the bacteria, and perform amplification and extraction to obtain the MOG plasmid;
[0051] S2: Lentiviral packaging that overexpresses the MOG gene, see Figure 7 An over...
Embodiment 2
[0070] A CBA detection kit for the detection of MOG autoimmune IgG antibody in clinical samples, the kit includes anti-human IgG-FITC secondary antibody, blocking solution, 10×PBS, anti-fluorescent bleaching mountant and MOG-IgG CBA detection Use the cell slide assembly; the blocking solution is preferably 10% goat serum.
[0071] Preferably, the cell slide assembly for MOG-IgG CBA detection is prepared from slides or slides containing small chambers, Vector-293T cells and MOG-293T cells, specifically, the slide containing small chambers contains 12 The detachable glass slides of chambers, wherein chamber 6 contains MOG-293T cells, and chamber 6 contains Vector-293T cells. For the specific preparation method, refer to step S4 of Example 1.
Embodiment 3
[0073] An application method of a CBA test kit, that is, the detection method using the test kit comprises the following steps:
[0074] Step1: The cell slide assembly for MOG-IgG CBA detection prepared in Example 1 or the cell slide assembly for MOG-IgG CBA detection described in Example 2 (abbreviated as cell slide assembly) can be stored at -80°C More than 12 months, store at -20°C for more than 1 month.
[0075] Step2: Take a clinical serum or cerebrospinal fluid sample. If it cannot be used immediately, it should be separated immediately and stored at -80°C to ensure the natural activity of the protein in the sample to the greatest extent;
[0076]Step3: According to the number of samples to be tested, determine the number of cell slide assemblies required. If no multiple wells are used, one cell slide assembly can detect 6 samples; if multiple wells are used, one cell slide assembly can detect 3 samples. .
[0077] Step4: Equilibrate the cryopreserved cell slide assemb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com