Cationic gene vector with high transfection efficiency and low cytotoxicity and preparation method thereof
A technology of transfection efficiency and cytotoxicity, applied in the field of new biomedical materials, can solve problems such as poor repeatability and complicated preparation methods, and achieve low cytotoxicity, high transfection efficiency, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention also provides a method for preparing a cationic gene carrier with high transfection efficiency and low cytotoxicity, comprising the following steps:
[0030] A) will C 8 h 17 N 3 .HCl and 1-hydroxybenzotriazole are added to the N,N-dimethylformamide solution of p-toluenesulfonyl and tert-butoxycarbonyl double-protected arginine for activation;
[0031] B) adding an aqueous solution of linear poly-α-lysine to the activated solution in step A) for reaction;
[0032] The molar ratio of the linear poly-α-lysine to p-toluenesulfonyl-protected arginine is 1: (5-120);
[0033] C) Dialyzing and freeze-drying the solution after the reaction in the step B) in sequence to obtain an intermediate product;
[0034] D) Reacting the intermediate product under the condition of trifluoroacetic acid, adding anhydrous ether for precipitation, vacuum drying and dialysis to obtain the cationic gene carrier.
[0035] In the present invention, arginine (Boc-Arg (Tos)...
Embodiment 1
[0050] The preparation of embodiment 1PLL-Arg (Tos)
[0051] Linear poly-α-lysine was dissolved in deionized water, and p-toluenesulfonyl and tert-butoxycarbonyl double-protected arginine (Boc-Arg(Tos)) was dissolved in DMF. Add EDC.HCl and HOBT to the Boc-Arg (Tos) solution to activate the reaction at room temperature for 1 h, then slowly add the aqueous solution of PLL to the above mixture, and react at room temperature for 72 h. The reaction mixture was dialyzed and lyophilized. Then the lyophilized product was reacted under the condition of trifluoroacetic acid for 4 h, concentrated in vacuo, settled by adding anhydrous ether, vacuum-dried, dialyzed and lyophilized to obtain the white solid product PLL-Arg(Tos).
[0052] The grafting ratios of poly-α-lysine grafted to tosyl-protected arginine (Arg(Tos)) are listed in Table 1.
[0053] Table 1 The corresponding relationship between the molar ratio of raw materials and the molecular weight of products
[0054] Ca...
Embodiment 2
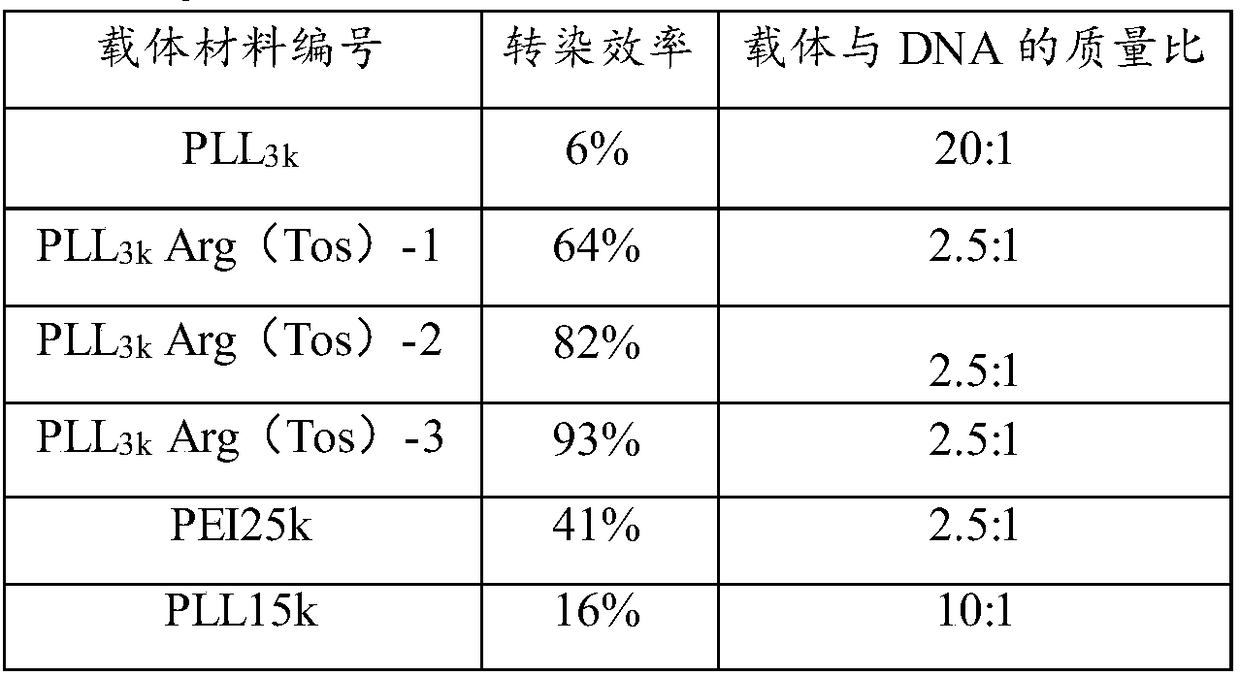
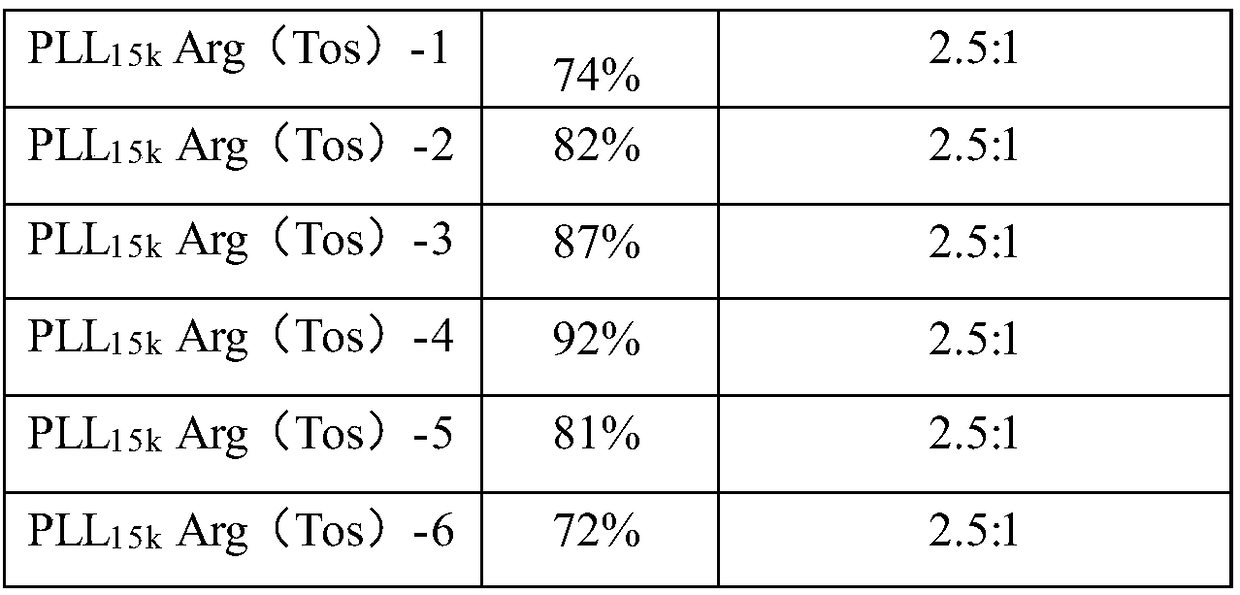
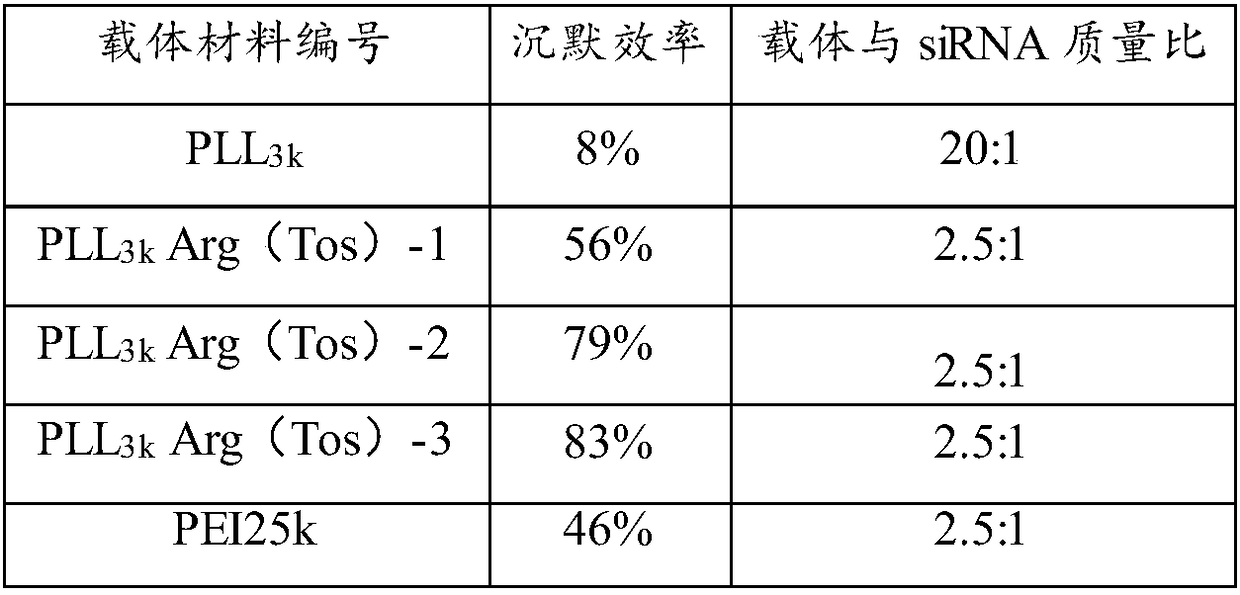
[0056] 1) Using PLL-Arg (Tos) to mediate the in vitro transfection of pGL3 (luciferase plasmid) and pEGFPN1 (green fluorescent protein plasmid) to MCF-7 cells
[0057] (1) Culture of MCF-7 cells
[0058] MCF-7 cells were cultured in fetal calf serum medium containing 10% volume fraction at a set temperature of 37°C and 5% volume fraction of CO 2 cultured in a constant temperature incubator.
[0059] (2) Cell transfection
[0060] 24 hours before transfection, cells in the logarithmic growth phase were taken, digested with trypsin, and diluted with 10% fetal calf serum culture medium, according to 1×10 4 The density of cells / well was plated in a 96-well cell culture plate, and placed in a culture temperature of 37°C with a volume fraction of 5% CO 2 Cultured in a constant temperature incubator until the cells reached 80-90% confluence. During transfection, after compounding the carrier / rhoDNA complex for 20 minutes, the amount of 0.2 μg ρDNA / well was added to the 96-well ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com