ATP releasing agent and germ fast detection reagent containing ATP releasing agent
A detection reagent and release agent technology, applied in the field of bacterial rapid detection reagents, can solve the problems of changing cell membrane permeability, enzymatic denaturation and inactivation, and insignificant ATP release effect, and achieves simple preparation process, rapid process, and reduced ATP release. effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, the construction of the recombinant expression vector containing luciferase gene
[0032] 1. Acquisition of luciferase gene
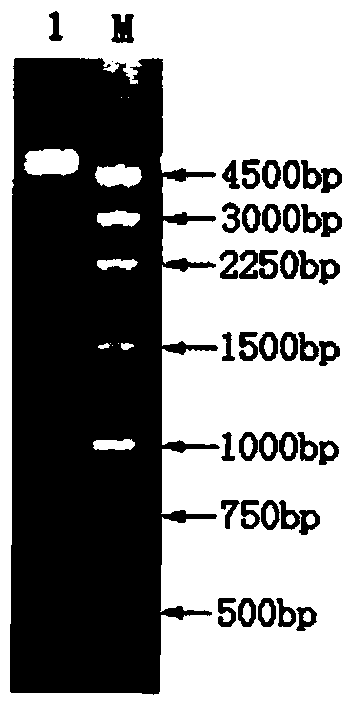
[0033]According to the Japanese firefly luciferase gene (GenBank: X66919.1), the whole gene synthesis method was adopted, and Shanghai Jierui Biological Co., Ltd. was entrusted to synthesize the whole gene, and at the same time, the protein structure was analyzed to affect the temperature of the enzyme without affecting the enzyme activity. The codons in the codon were mutated, and the 217 codon GCA encoding Ala was changed to CTT encoding Leu, the 457 codon GAA encoding Glu was changed to TCA encoding Ser, and the 465 codon AAT encoding Asn was changed to ATT encoding Ile , and add CAT and AAGCTT to its 5' and 3' ends respectively to obtain the enzyme-cleavage recognition sites CATATG and AAGCTT of endonuclease NdeI and Hind III respectively, the sequence length is 1656 bp, and the rare codons in bacteria will be replaced with bact...
Embodiment 2
[0038] Example 2. Induced expression and expanded culture of luciferase
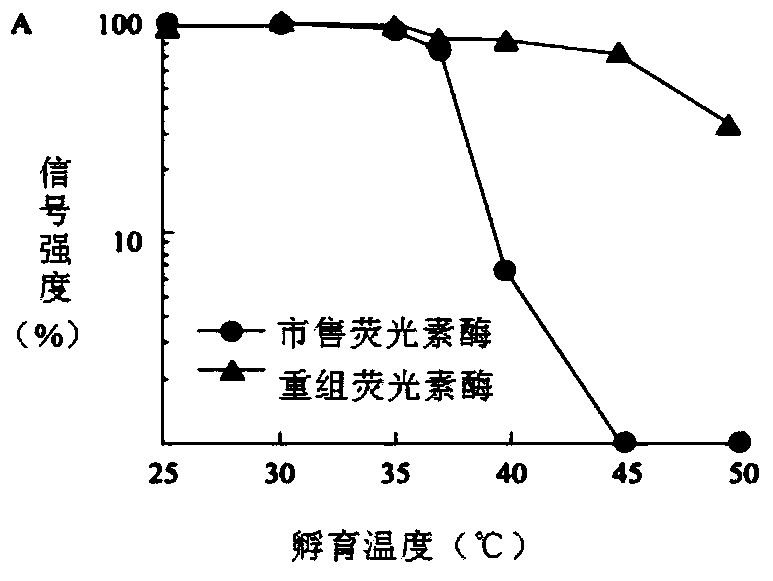
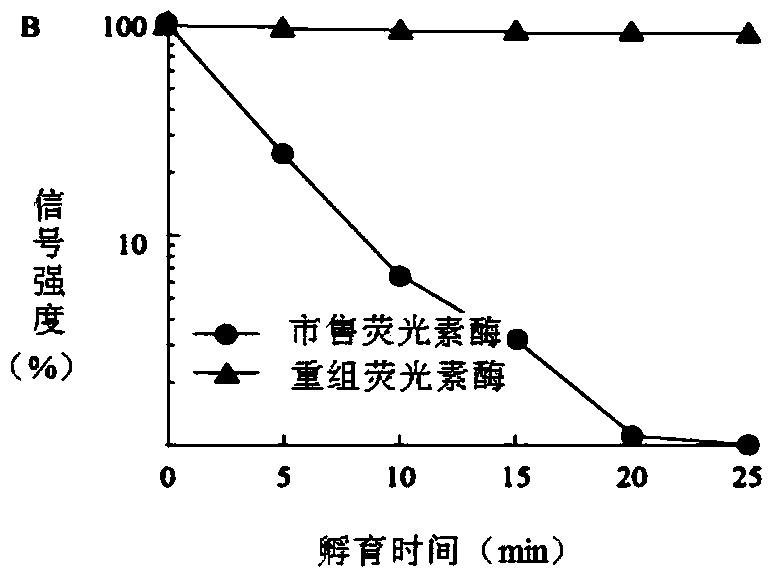
[0039] Get the recombinant expression vector pET28a-rt-L1L that embodiment 1 obtains and transfer Escherichia coli BL21 (DE3) competent cell, cultivate on the LB culture plate that contains kanamycin, select single bacterium colony and inoculate in 5mlLB medium and screen and obtain The positive recombinant bacteria were induced to express, and the product was analyzed by SDS-PAGE, and compared with the control bacteria containing the expression vector pET28a(+), the results were as follows Figure 4 shown in the instruction manual Figure 4 Shown is the induced expression SDS-PAGE protein electrophoresis pattern of recombinant firefly luciferase, in which there are many foreign proteins.
[0040] The bacterial supernatant and total protein of the control bacteria without the target gene ( Figure 4 Compared with lanes 3 and 4 in ), the recombinant protein band with a molecular weight of about 60 kDa c...
Embodiment 3
[0042] Embodiment three, the separation and purification of luciferase
[0043] Since the recombinant expression vector pET28a-rt-L1L constructed in the above-mentioned Example 1 has a fusion tag (His·Tag) gene sequence, the expressed target protein also has a His·Tag tag. Therefore, the target protein can be purified by affinity chromatography using agarose complexed with Ni, and the affinity chromatography column is purchased from GE Healthcare.
[0044] After the expression was induced with IPTG according to the method of the above-mentioned Example 2, the bacterial cell pellet was harvested by centrifugation at 10000 rpm / min for 5 min; L cells) were resuspended, ultrasonically lysed at 250 W 90 times, each time for 5 s, with an interval of 10 s, and centrifuged at 12,000 rpm / min for 20 min at 4°C to collect the supernatant and prepare for loading.
[0045] The specific purification steps are as follows:
[0046] 1) Equilibrate the Ni column with Buffer A until the OD280 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com