Photosensitive compound, preparation method and application thereof, and photosensitive protein immobilized gel containing photosensitive compound
A compound and photosensitivity technology, applied in the field of chemical biology, can solve the problems of low sensitivity, inactivation of protein antigenic sites, short excitation wavelength, etc., and achieve the effects of simple operation, stable protein fixation and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
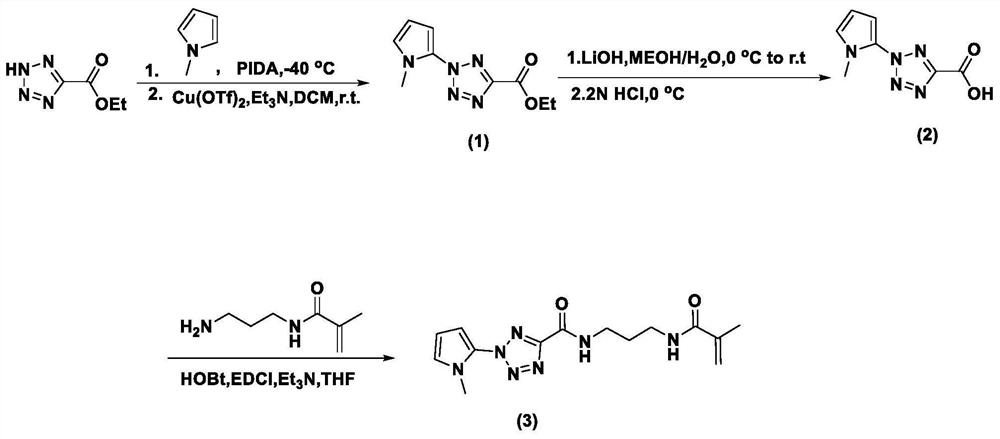
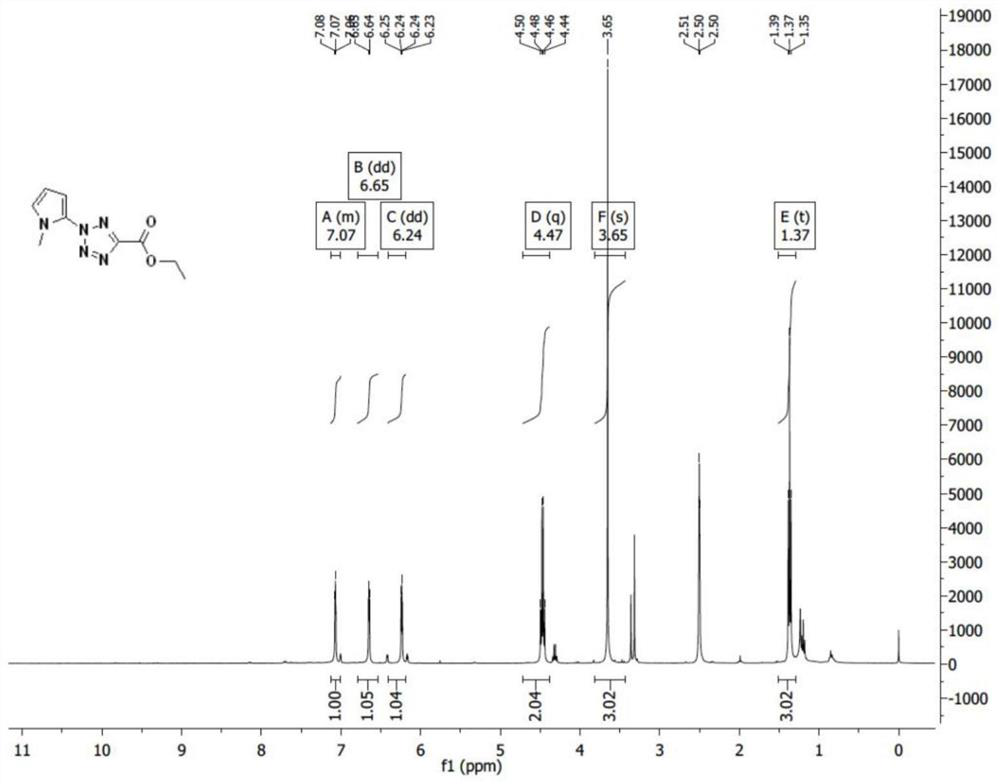
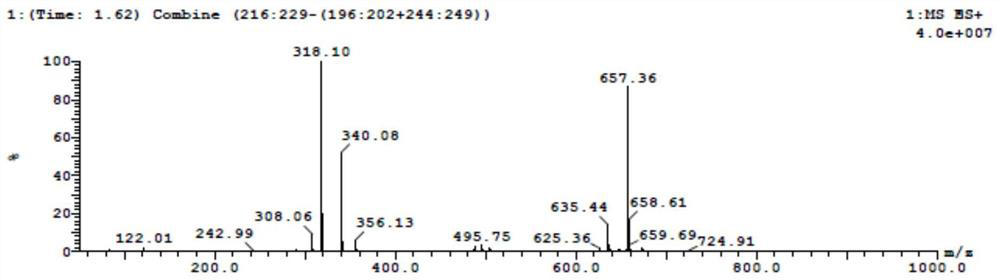
[0060] According to the molar ratio of 1:11:200, weigh methyl-1 hydrogen-pyrrole, iodobenzene diacetate, and trifluoroethanol, first dissolve methyl-1 hydrogen-pyrrole in trifluoroethanol, and then Add iodobenzene diacetate and stir for 3h under nitrogen protection. The stirred mixture was concentrated to a black oil which was dissolved in dichloromethane. Then, 5-ethyl tetrazolium carboxylate, copper (II) trifluoromethanesulfonate and triethylamine weighed in a molar ratio of 3:1:13 were added thereto; stirred at room temperature for 24 h under nitrogen protection. The obtained substance was washed with saturated ammonium chloride and brine respectively, then dried with anhydrous magnesium sulfate, finally filtered, and further purified by silica gel chromatography (eluent: PE:EA=8:1) to obtain a brown oily intermediate Product, its structural formula is shown in following formula (1).
[0061]
[0062] Wherein, the yield rate of the above preparation method is 9.6%. Su...
Embodiment 2
[0070] A photosensitive compound whose structural formula is shown in the following formula (4) is prepared.
[0071]
[0072] Weigh pyrrole, iodobenzene diacetate and trifluoroethanol according to the molar ratio of 1:10:170, first dissolve pyrrole in trifluoroethanol, then add iodobenzene diacetate at –40°C, and stir for 3 h under nitrogen protection. The stirred mixture was concentrated to a black oil which was dissolved in dichloromethane. Then, 5-ethyl tetrazolium carboxylate, copper (II) trifluoromethanesulfonate and triethylamine weighed according to the molar ratio of 4:1:15 were added thereto; stirred at room temperature for 24 h under nitrogen protection. The obtained substance was washed with saturated ammonium chloride and brine respectively, dried with anhydrous magnesium sulfate, filtered, and further purified by silica gel chromatography (eluent: PE:EA=7:1) to obtain a brown oily intermediate product.
[0073] Weigh the intermediate product and lithium hydr...
Embodiment 3
[0076] A photosensitive compound whose structural formula is shown in the following formula (5) is prepared.
[0077]
[0078] Weigh thiophene, iodobenzene diacetate and trifluoroethanol according to the molar ratio of 1:12:150, first dissolve thiophene in trifluoroethanol, then add iodobenzene diacetate at –40°C, and stir for 4 h under nitrogen protection. After the stirred mixture was concentrated to a black oil, it was dissolved in dichloromethane. Then, 5-ethyl tetrazolium carboxylate, copper (II) trifluoromethanesulfonate and triethylamine weighed according to the molar ratio of 5:1:16 were added thereto; stirred at room temperature for 27 h under nitrogen protection. The obtained material was washed with saturated ammonium chloride, dried over anhydrous magnesium sulfate, finally filtered, and further purified by silica gel chromatography (eluent: PE:EA=6:1) to obtain a brown oily intermediate product.
[0079] Weigh the intermediate product and lithium hydroxide accor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com