Methods related to immunostimulatory nucleic acid-induced interferon
a nucleic acid-induced interferon and immunostimulatory technology, applied in the direction of antibody medical ingredients, drug compositions, peptide/protein ingredients, etc., can solve the problem of limited therapeutic use of ifn- treatment, and achieve the effect of preventing an ifn- treatment-related side
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Characterization of IPCs
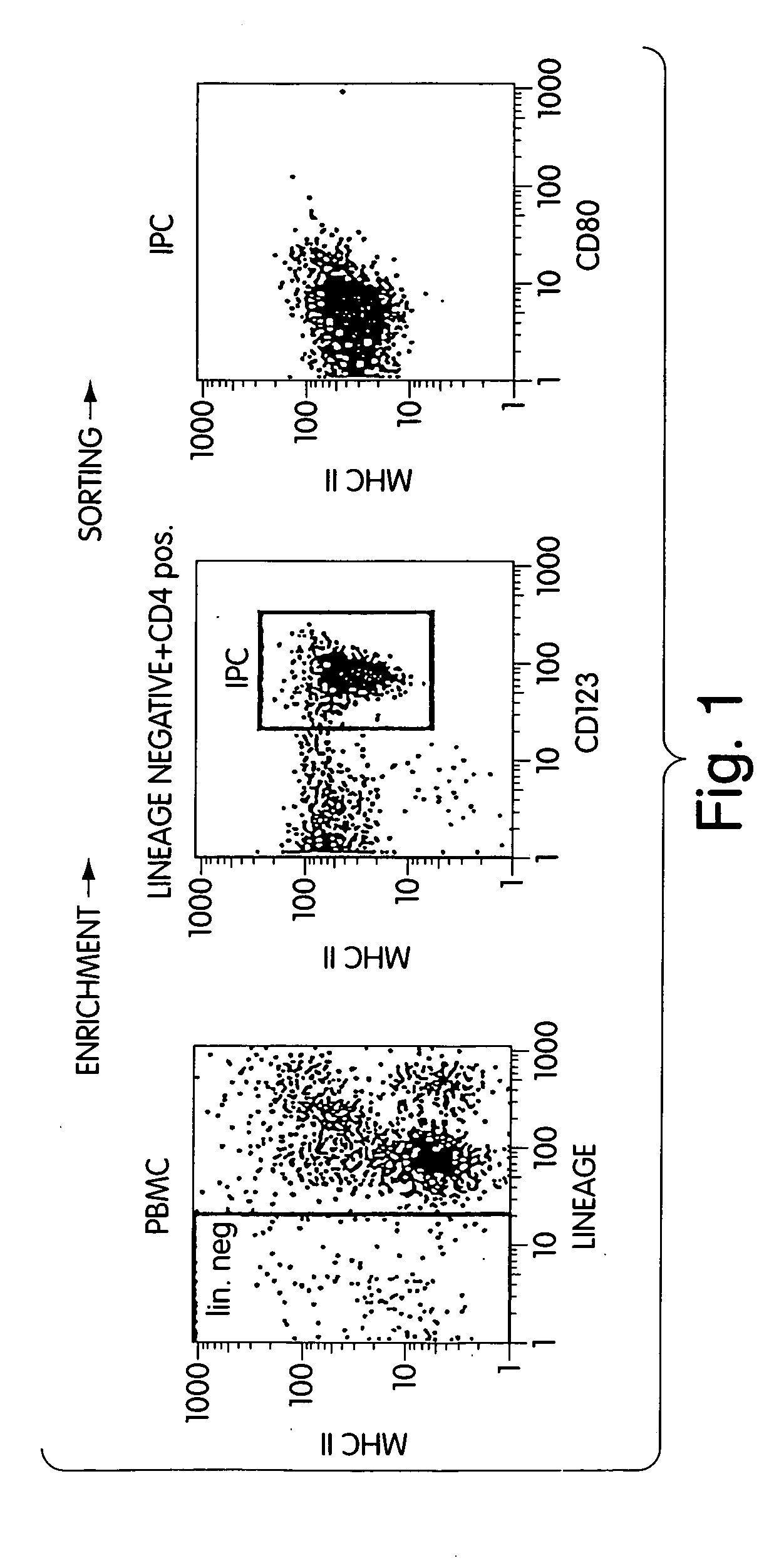
[0172] Peripheral blood mononuclear cells (PBMCs) contain a total of 0.2 to 0.4 percent IPCs, which are characterized by the lack of lineage markers (CD3, CD14, CD16, CD19, CD20, CD56) and can be distinguished from other lineage-negative cells by the expression of CD4, CD123 (IL-3Rα), and MHC class II.
[0173] IPCs were isolated from peripheral blood by using the VARIOMACS technique (Milteny Biotec, Auburn, Calif.) and the technique previously described. O'Doherty U et al. J Exp Med 178:1067-76 (1993). PBMCs were obtained from buffy coats of healthy blood donors by Ficoll-Paque density gradient centrifugation (Histopaque-1077, Sigma) as previously described. Hartmann G et al. Antisense Nucleic Acid Drug Dev 6:291-9 (1996). Monoclonal antibodies directed to CD3 (UCHT1), CD14 (M5E2), and CD19 (B43) were purchased from PharMingen (San Diego). PBMCs were incubated with anti-CD3, CD14, CD16, CD19, and CD56 antibodies conjugated to colloidal superpara...
example 2
CpG Oligonucleotide Supports the Survival and Activation of IPCs In Vitro
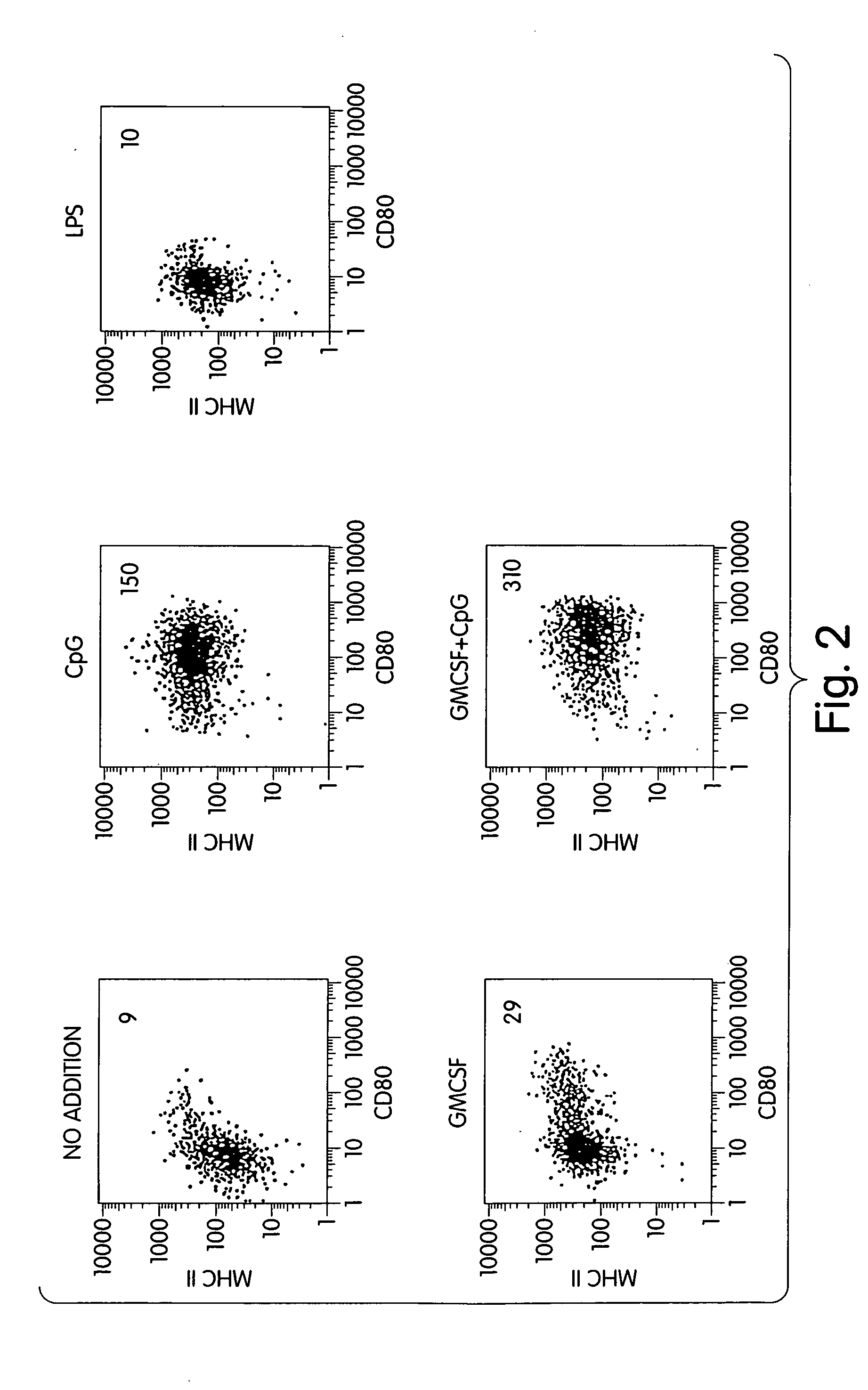
[0177] The majority of freshly isolated IPCs die within 3 days if not incubated in the presence of IL-3 or GM-CSF. Remaining live cells are not activated or are only weakly activated. If CpG oligonucleotide but no other growth factors are added to the cell culture of IPCs, IPCs survive and become highly activated as shown by their increased expression of costimulatory molecules (e.g., CD80, FIG. 2).
[0178] Freshly isolated IPCs (see Example 1) were suspended in RPMI 1640 culture medium supplemented with 10 percent (vol / vol) heat-inactivated (56° C., 1 h) FCS (HyClone), 1.5 mM L-glutamine, 100 units / ml penicillin, and 100 μg / ml streptomycin (all from GIBCO / BRL) (complete medium). All compounds were purchased endotoxin-tested. Freshly prepared IPCs (final concentration 5×105 cells per ml) were cultured for two days in complete medium alone or complete medium supplemented with 6 μg / ml phosphorothioate CpG ODN 200...
example 3
CpG Oligonucleotide, but not Poly IC, Activates IPCs In Vitro
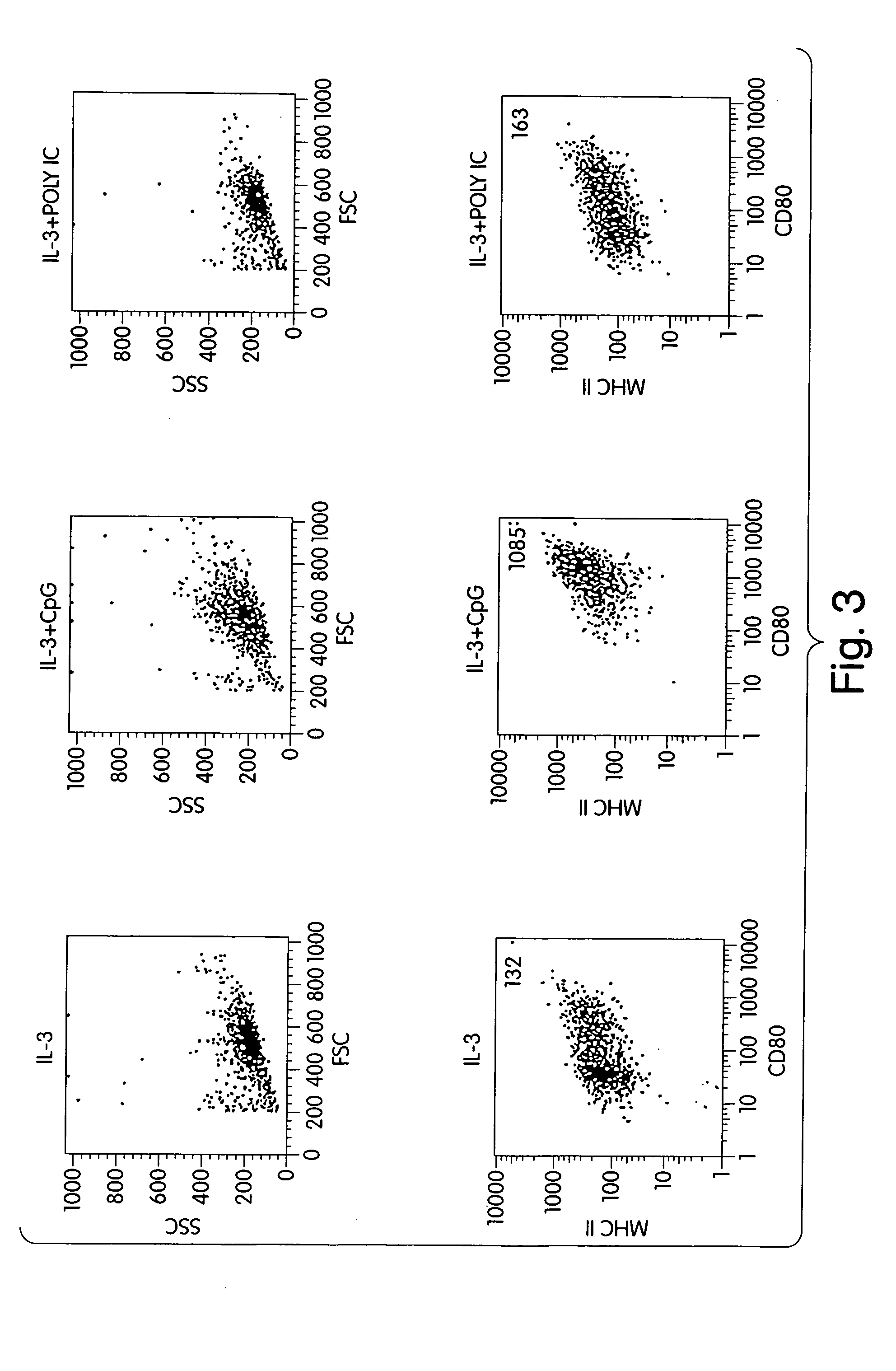
[0180] IL-3 provides excellent survival of IPCs but does not activate IPCs. When IL-3 was combined with CpG oligonucleotide, expression of CD80 increased by 5 to 20-fold (FIG. 3). Poly IC, another polynucleotide with well known immunostimulatory functions on myeloid cells (dendritic cells, macrophages), did not stimulate IPCs.
[0181] Freshly prepared IPCs (see Example 1, final concentration 3×105 cells per ml) were cultured for three days in complete medium (see Example 2) supplemented with 10 ng / ml IL-3. Cultures of IPCs were then continued for a further 24 hours (a) without any additional supplements, (b) following the addition of 6 μg / ml CpG ODN 2006 (SEQ ID NO:147), and (c) following the addition of 10 μg / ml poly IC. Forward scattering (FSC), side scattering (SSC), and expression of CD80 and MHC class II on IPCs were examined by flow cytometry (see Example 1).
[0182] Results. Representative results of three independen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com