Dressing device for use wtih a cannula or a catheter
a dressing device and cannula technology, applied in the field of wound devices, can solve the problems of adversely affecting wound healing, not the case, and achieve the effect of not adversely affecting the function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Haemostatic and Antimicrobial Polyurethane Foam Preparations
[0049]To prepare haemostatic and antimicrobial foam the haemostat polyanhydroglucuronic acid (PAGA) (HemCon Medical Technologies Europe Ltd, Dublin) and the antimicrobial compound chlorhexidine di-gluconate (CHG) (Kapp Technologies LLC, New Jersey) were used. Polyurethane foam dressings were prepared with varying concentrations of PAGA and CHG relative to the final dry weight of polyurethane foam. The polyurethane foam used is type MS50P(w) Lendell medical foam available from Filtrona Porous Technologies (www.filtronaporoustechnologies.com)
Usable Width:15 inches (381 mm)Thickness:0.22 inches (5.6 mm)% Moisture:2%Density:6.0 pcf (96 Kg / m3)Tensile Strength:51.0 psi (352 kPa)Target Elongation:194%Tear Strength:5.6 pli (0.98 kN / m)CDF @ 50%:0.74 psi (5.14 kPa)Durometer:47 shoreCell Size:131 ppiAbsorption:15 g / gExpansion:75%Wrung Retention:1.2 g / g
[0050]The polyurethane foam was produced by firstly producing a prepolymer comprisin...
example 2
Antibacterial Efficacy of Prepared Formulations Calcium Sodium Salt polyanhydroglucuronic Acid and Chlorhexidine di-gluconate in a polyurethane Foam
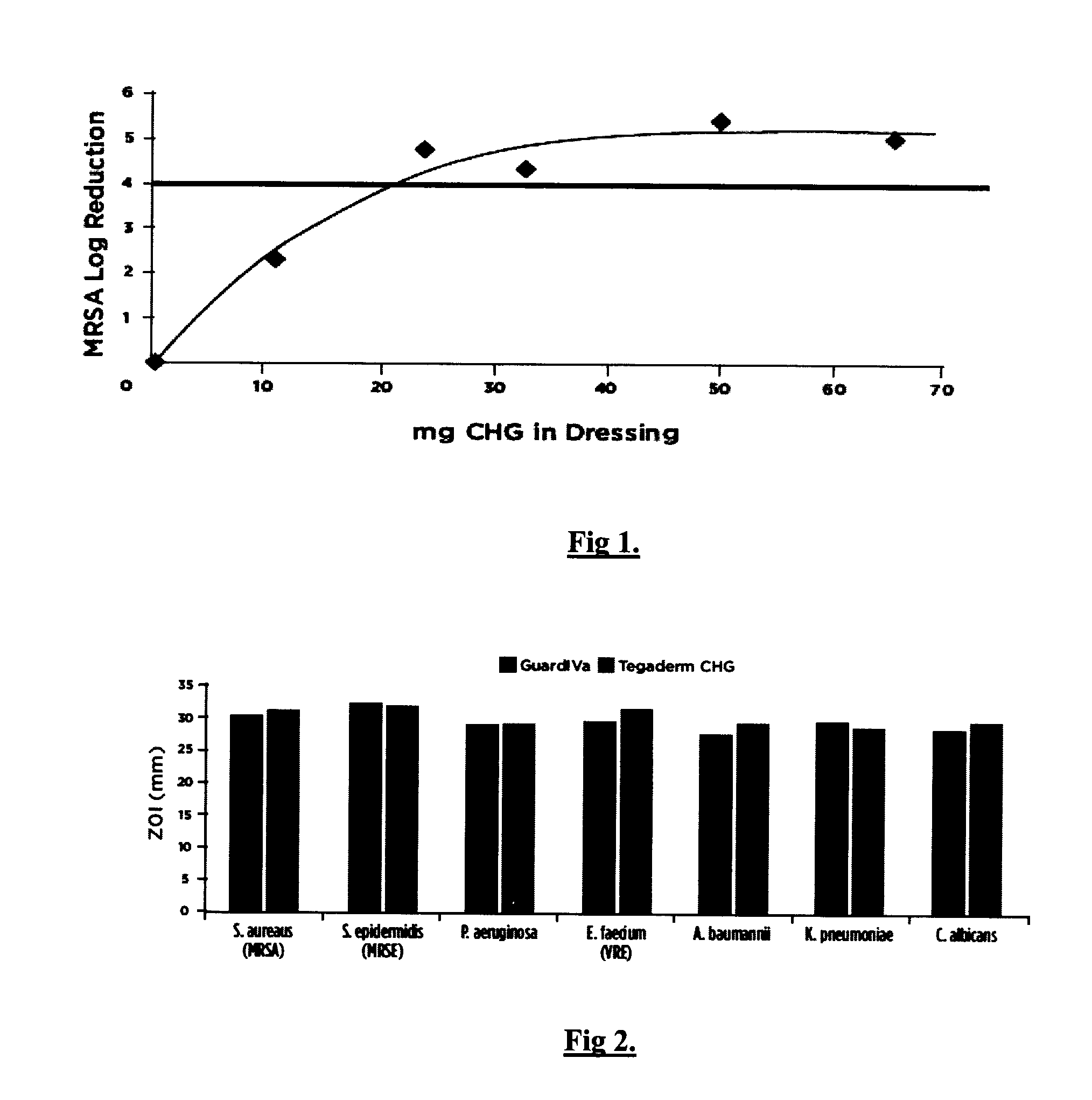
[0051]Polyurethane foam matrix dressings were prepared with the calcium sodium salt of polyanhydroglucuronic acid (15% w / w) and w / w percentages of CHG at 0%, 5%, 11%, 15%, 23% and 30% as presented in Example 1. These formulations were investigated for their antibacterial efficacy against methicillin-resistant Staphylococcus aureus (MRSA) using AATCC Test Method 100 “Assessment of Antibacterial Finishes on Textiles”.
[0052]Analysis of FIG. 1 indicates that the acceptable minimum low range of chlorhexidine di- gluconate percentage weight fraction in the polyurethane foam matrix is 9% (20 mg) to 16% (35 mg) w / w since this range achieves the acceptable >Log 4 reduction.
[0053]The results for gamma-irradiated sterilized testing and non gamma-irradiated testing are presented in Table 2.
TABLE 2Formulations of PAGA impregnated PU foam with increas...
example 3
Device Assembly
[0054]A catheter access site dressing device to control bleeding was prepared by impregnating calcium sodium salt of polyanhydroglucuronic acid into polyurethane foam. CHG was incorporated to achieve an antimicrobial efficacy of greater than 4 log in 24 hours. A formulation as described in Table 3 was prepared and a moisture vapour permeable backing that comprised of polyurethane film with a MVTR of 1000 gm / m2 / 24 hr (3M) was adhered.
TABLE 3IV site device compositionFormulation (% w / w)Ingredientsfinal formulationChlorhexidine gluconate11Calcium-sodium polyanhydroglucuronic acid8Hydrophillic flexible polyurethane foam81
[0055]The polyurethane foam matrix was die cut into 25. mm diameter disks with a central 4 mm diameter section removed from each disk. A radial slit was also punched from the centre of the disk to the outside of the disk. The slit and 4 mm punch are designed to allow catheter access. The dressing is sterilized by gamma irradiation between 25 and 45 kGy, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com