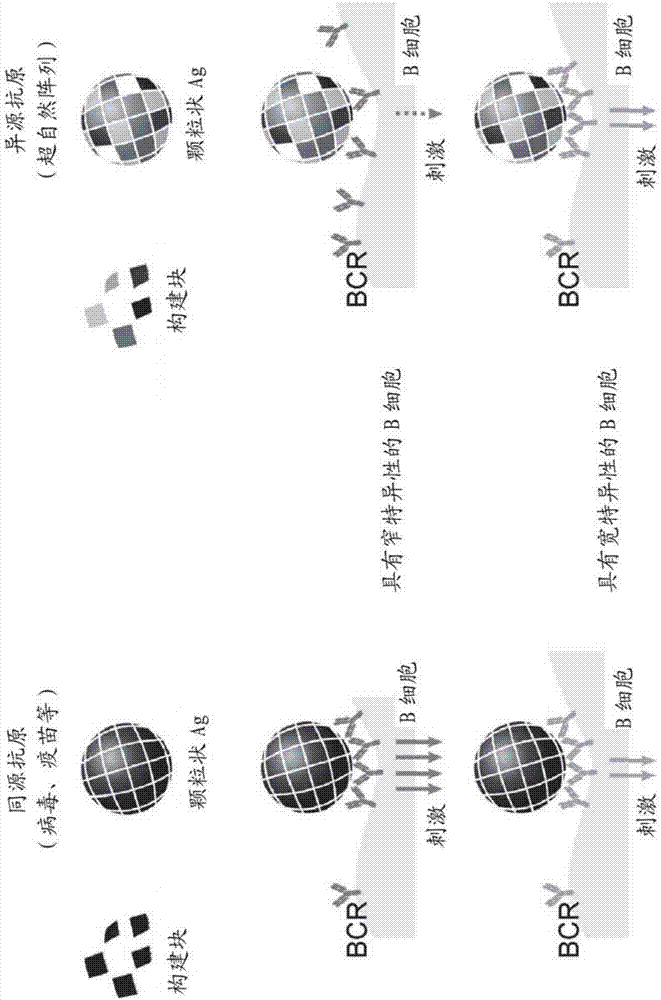
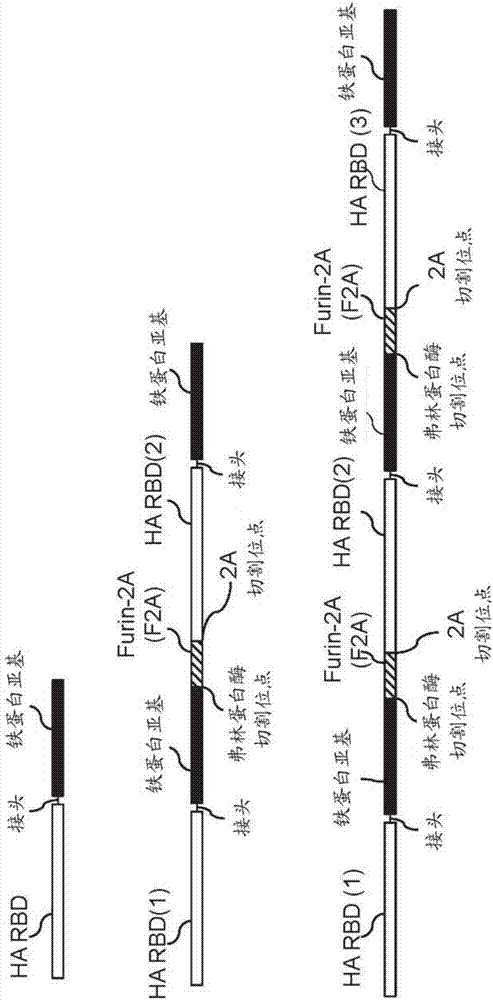
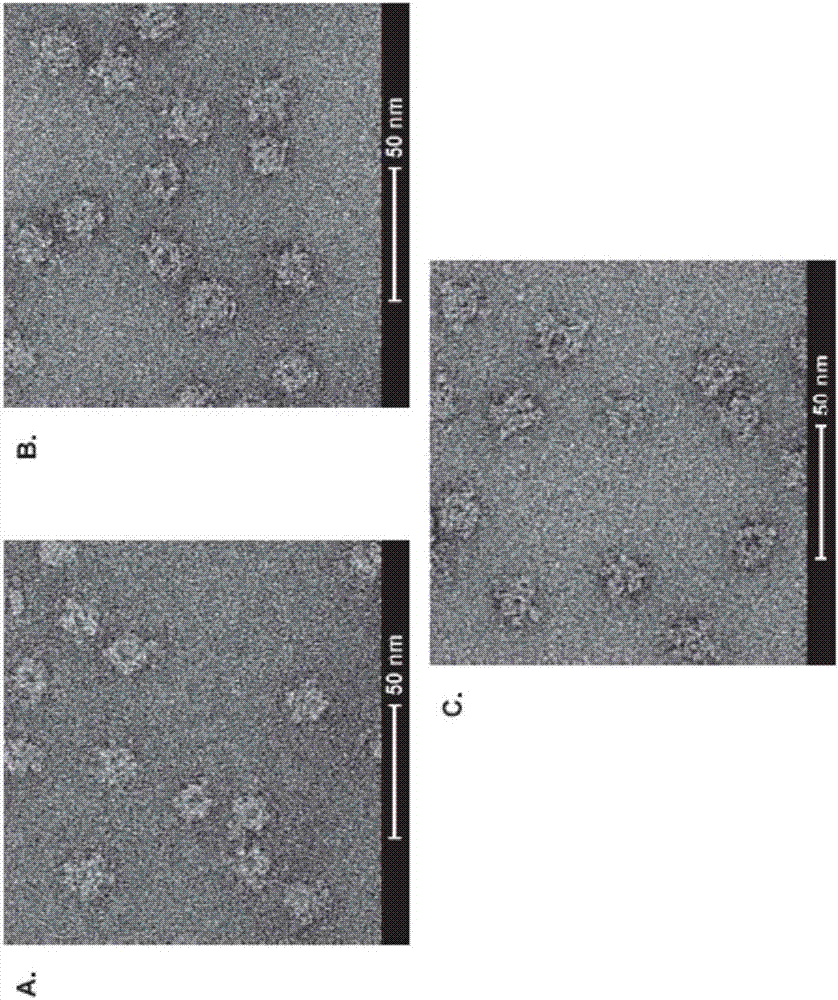
Novel multivalent nanoparticle-based vaccines
A nanoparticle and subunit technology, applied in the field of new multivalent vaccines based on nanoparticles, can solve problems such as antibody difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: Production of heterogeneous nanoparticles
[0168] A. Gene synthesis and vector construction
[0169] All genes used in the study were human codon optimized. The gene encoding the H. pylori-bullfrog hybrid ferritin was constructed by incorporating a point mutation (N8Q) at residue 8 of the bullfrog (Rana catesbeiana) ferritin lower subunit (UniProt: P07797) to eliminate potential N-glycosylation site) residues 2-9 with Helicobacter pylori non-heme ferritin (UniProt: Q9ZLI1, residues 3-167) (at residue 7 (I7E) and residue 19 ( N19Q) has mutations to form a salt bridge and eliminate a potential N-glycosylation site) fusion to 6R of bullfrog ferritin, respectively. In some cases, there is an additional GS residue at the carboxyl terminus of H. pylori ferritin. A secreted encapsulin gene was constructed by fusing the human CD5 signal to Thermotoga maritima encapsulin (UniProt:Q9WZP2, residues 1-264). The gene encoding the HA RBD (residues 56-264, H3 numbering...
Embodiment 2
[0174] Example 2: Immunoprecipitation analysis of purified nanoparticles expressing influenza HA protein RBD
[0175] HA RBD nanoparticles expressing RBD from NC99, CA09 or both (CoAsmbl 2) were prepared and purified as described in Example 1 . 4 μg of purified RBD nanoparticles were incubated with 4 μg of anti-NC99 (3u-u), anti-pandemic H1N1HA (2D1) or anti-HA stem region (C179) monoclonal antibodies for 30 min at room temperature. The immune complexes were then captured using protein G-coupled magnetic beads and washed extensively with PBS containing 0.01% Tween 20. The washed pellet was resuspended in 20 μl of Laemmli buffer without reducing agent and analyzed on SDS-PAGE. 5 μg of each protein was loaded. NC99, Type A / New Caledonia / 20 / 1999; CA09, Type A / California / 04 / 2009; WS33, Type A / Wilson-Smith / 1933; AB48, Type A / Albany / 4835 / 1948; BR07 , Type A / Brisbane / 59 / 2007; IA43, Type A / Iowa / 1943; HK77, Type A / Hong Kong / 117 / 1977; FM47, Type A / Fort Monmouth / 1 / 1947. The results o...
Embodiment 3
[0176] Example 3: Immunization of Mice Using Purified Nanoparticles
[0177] The ability of compositions comprising purified monovalent, mixed or heterogeneous nanoparticles to elicit a neutralizing immune response was tested in mice. BALB / c mice aged 6 to 8 weeks were divided into 9 groups (N=5). In the presence of the Sigma Adjuvant System (SAS), at weeks 0 and 3 each group was administered a composition comprising 2 μg of: a) monovalent (i.e., expressing a single HA RBD) nanoparticles, b) various nanoparticles Mixtures of particles, or co-assembled nanoparticles as indicated in c). The administered immunogens and dosing regimens are shown in Table 3 below.
[0178] table 3
[0179]
[0180] 1 Balb / c mice (N=5)
[0181] 2 Type A / New Caledonia / 20 / 99(NC99); Type A / California / 04 / 09(CA09); Type A / Wilson-Smith / 33(WS33); Type A / Albany / 4835 / 48( AB48); Type A / Brisbane / 59 / 07(BR07); Type A / Iowa / 43(IA43); Type A / Hong Kong / 117 / 77(HK77); Type A / Fort Monmouth / 1 / 47 (FM47)
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com