Composition resisting porcine epidemic diarrhea virus infection and application thereof
A technology for porcine epidemic diarrhea and virus infection, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, inorganic active ingredients, etc., can solve the problems of undisclosed glycerol monolaurate, and achieve the protection of intestinal epithelial mucosal layer, The effect of overcoming immune failure and inhibiting the replication of PEDV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
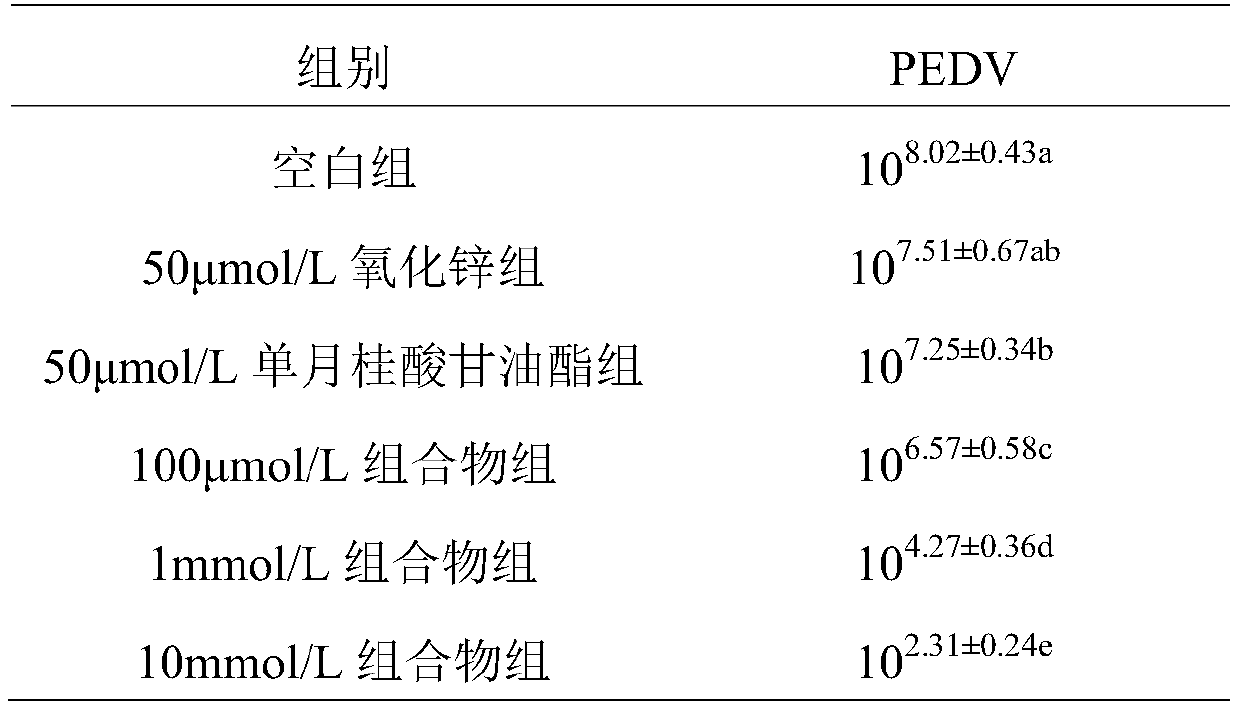
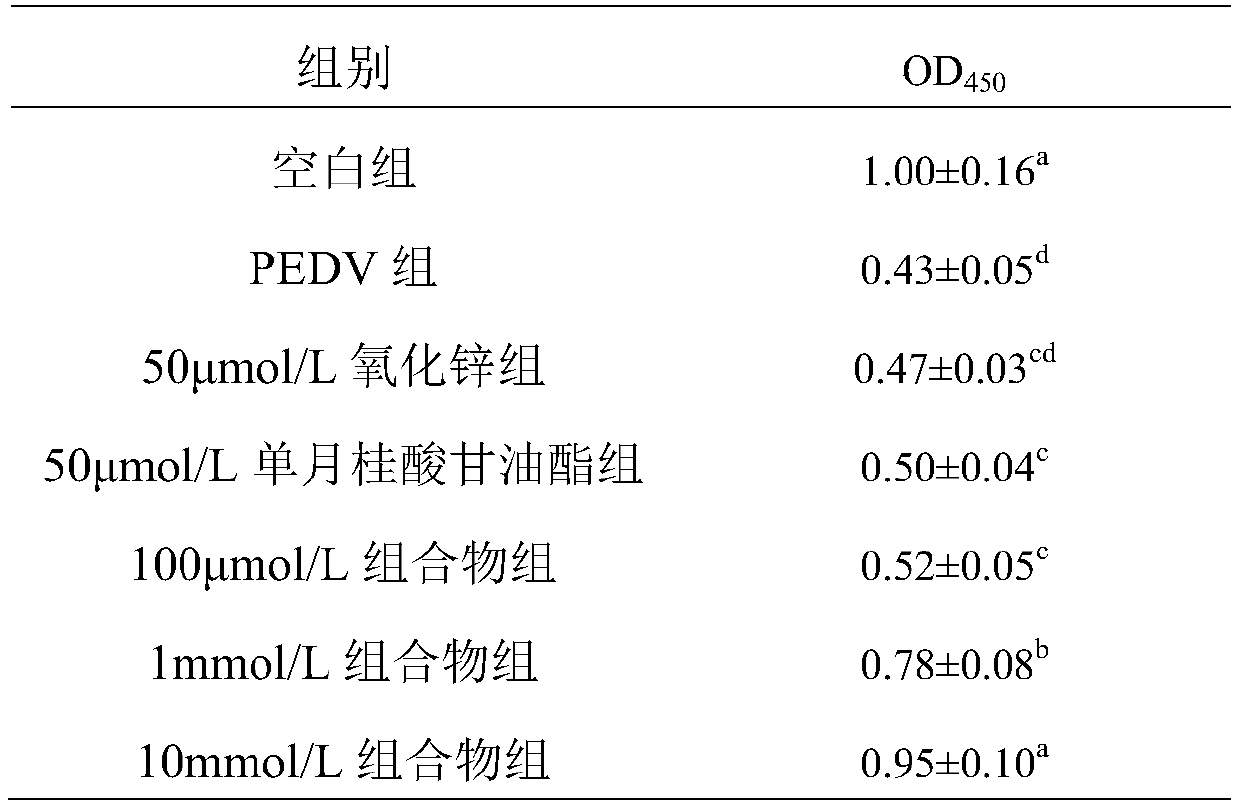
[0018] Inhibitory effect of zinc oxide and monolaurin composition on the proliferation of porcine epidemic diarrhea virus (PEDV) in vitro
[0019] (1) Test materials: African green monkey kidney cells (Vero cells) are used for the cultivation of porcine epidemic diarrhea virus, PED virus (YN strain) is isolated, preserved and gifted by the National Agricultural Microbiology Key Laboratory of Huazhong Agricultural University; DMEM medium EDTA, pancreatin, fetal bovine serum and double antibodies (penicillin) were purchased from GIBCO, zinc oxide and monolaurin were purchased from Sigma.
[0020] (2) Test method: Divide the test into three test groups, a blank group, a zinc oxide group and a monolauric acid glyceride group. The test groups were added to the culture medium with different doses of composition (oxidized in the composition). The weight ratio of zinc to monolauric acid glyceride is 1:1), in which the low-dose composition group (added according to 100 μmol / L medium), the d...
Embodiment 2
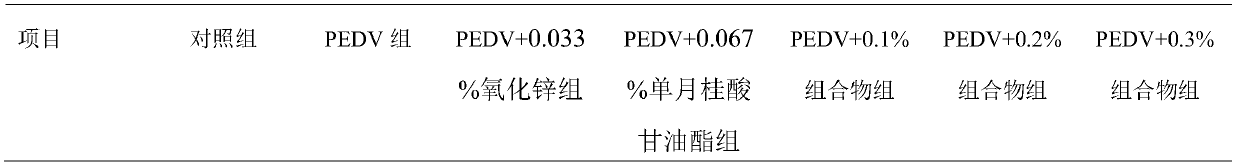
[0032] Protective effect of zinc oxide and monolaurin composition on piglets infected by PEDV
[0033] (1) Materials and methods: 210 21-day-old weaned piglets with the same weight and sex at one weaning were randomly divided into 7 groups: control group, PEDV group, PEDV+0.033% zinc oxide group, PEDV+0.067% single laurel Acid glyceride group, PEDV+0.1% composition group (the weight ratio of zinc oxide to glyceryl monolaurate is 0.5:1), PEDV+0.2% composition group (the weight ratio of zinc oxide to glyceryl monolaurate is 2:1), PEDV+0.3% composition group (weight ratio of zinc oxide to glycerol monolaurate is 2:1). 3 replicates (columns) per group, 10 heads per column. The basal rations for all groups were the same. The piglets in the PEDV+0.03% zinc oxide group and the PEDV+0.07% monolaurin group were fed a basal diet containing 0.033% zinc oxide and 0.067% monolaurin, respectively. The piglets of the PEDV+0.1% composition group, the PEDV+0.2% composition group and the PEDV+0...
Embodiment 3
[0050] A composition for resisting porcine epidemic diarrhea virus infection and its application, and its steps are:
[0051] (1) Weigh 10g of glycerol monolaurate and 30g of montmorillonite and mix well;
[0052] (2) Weigh 20g of zinc oxide and add it to the mixture in step (1), and mix well;
[0053] (3) Obtain a mixture against porcine epidemic diarrhea virus infection (mass ratio of composition to carrier = 1:1), wherein the weight ratio of zinc oxide to glyceryl monolaurate in the composition is 2:1;
[0054] (4) As the usual prevention of swine epidemic diarrhea virus infection, the mixture is added to the feed at 0.2% of the total weight of the feed (the actual additive dose of the composition of monolaurin and zinc oxide is 0.1%). Used throughout the feeding cycle;
[0055] (5) When used for clinical swine epidemic diarrhea virus infection treatment, the mixture can be added to the feed at a dose of 0.6% of the total weight of the feed (the actual additive dose of the compositi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com