Peroxide removal from drug delivery vehicle
a technology for drug delivery and peroxide, which is applied in the direction of drug compositions, peptide/protein ingredients, inorganic non-active ingredients, etc., can solve the problems of drug delivery system, delivery system, and active ingredient oxidation in the formulation, so as to prolong the stability of the drug, and reduce the level of peroxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
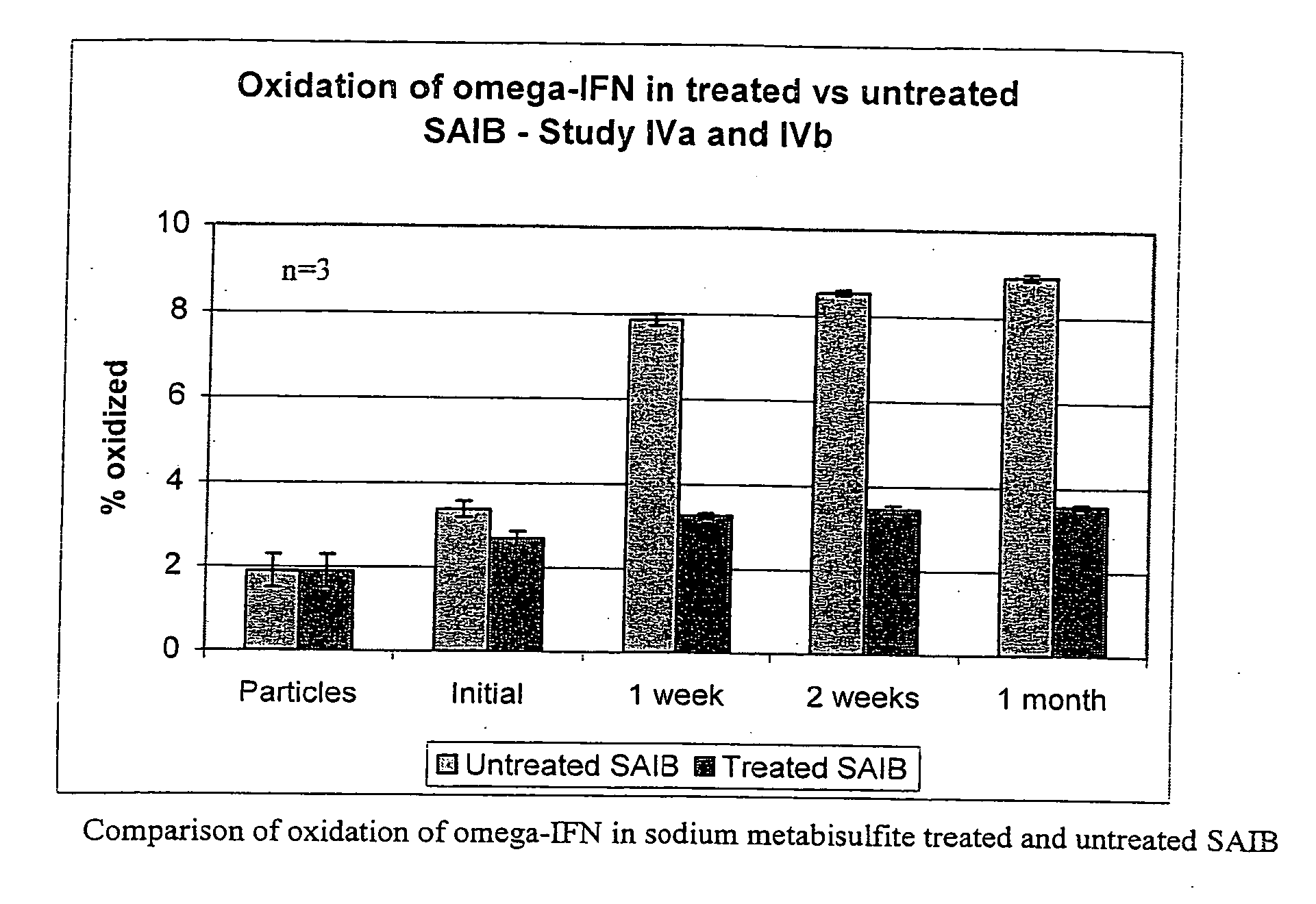
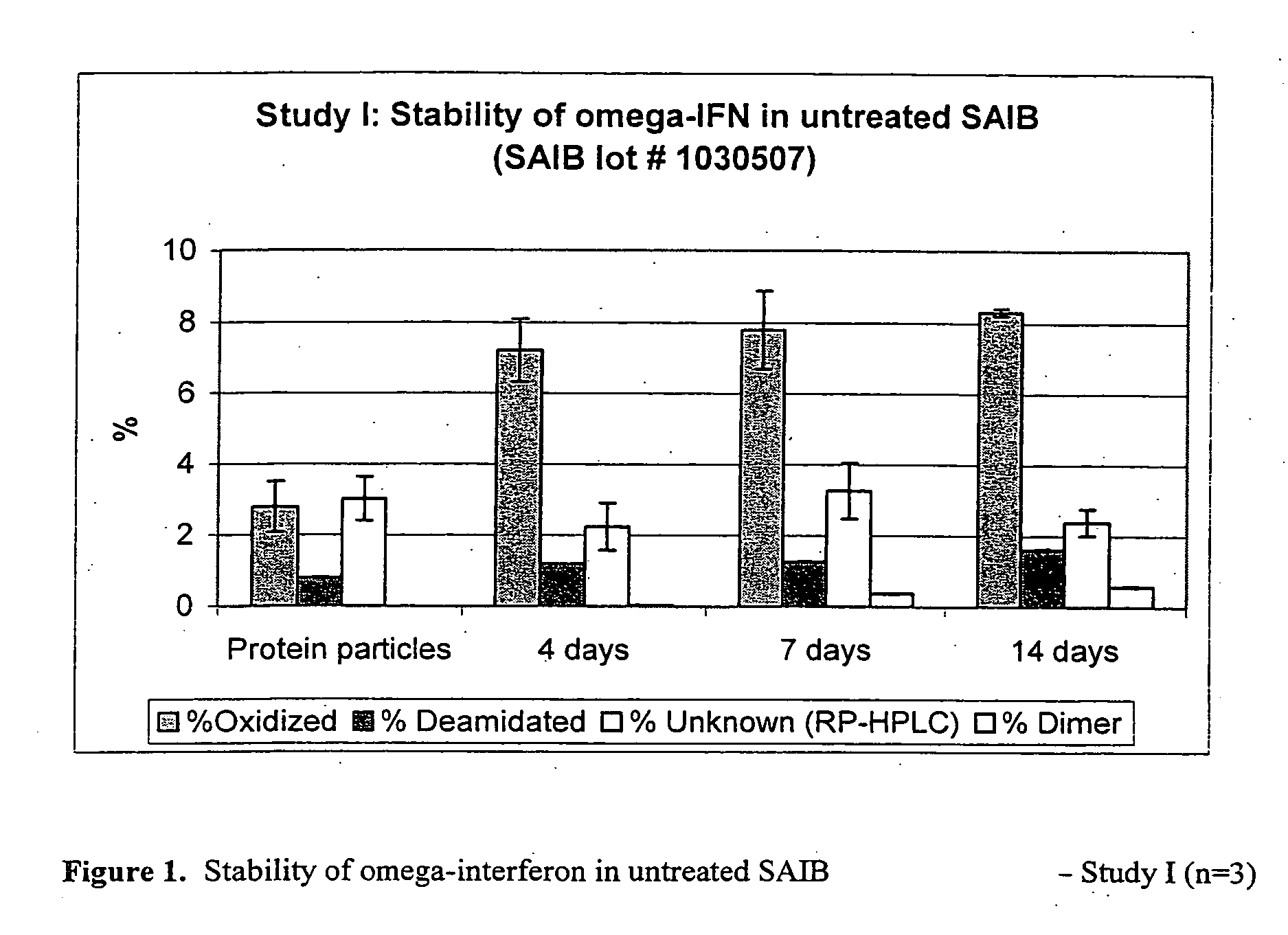
[0042] Study I: Stability in Untreated SAIB (Lot #TD1030507) for 2 Weeks
TABLE 4Stability of omega-interferon in untreated SAIB (lot #: 1030507) - Study IAnalysis by RP-HPLC (n = 3)**Initial (t = 0)(AR 48452)4 days7 days14 days(protein particles)***AR48424AR48562AR48450Assay (%)NA0.59* (0.02) 0.72 (0.00)0.68 (0.00)% omega-IFN 93.37 (0.40)89.06 (0.46) 87.65 (0.06) 87.67 (0.26) Purity% Oxidized 2.8 (0.71)7.21 (0.88)7.79 (1.09)8.31 (0.10)% Deamidated 0.8 (0.02)1.21 (0.00)1.28 (0.01)1.63 (0.03)% Unknown 3.03 (0.62)2.25 (0.66)3.27 (0.79)2.39 (0.38)Analysis by SEC (n = 3)**Initial(AR 48452)4 days7 days14 days(protein particles)***AR48424AR48562AR48450% Monomer100.00 (0.00)99.96 (0.01) 99.60 (0.02) 99.40 (0.00) % DimerND0.04 (0.00)0.38 (0.01)0.58 (0.02)UnknownNDND0.01 (0.00)0.01 (0.01)
ND = Not detected,
*sampled by scraping container walls, so values might not be representative of the bulk
**standard deviation in parenthesis;
***protein particles - t = 0 for suspension
[0043] The prelim...
example 2
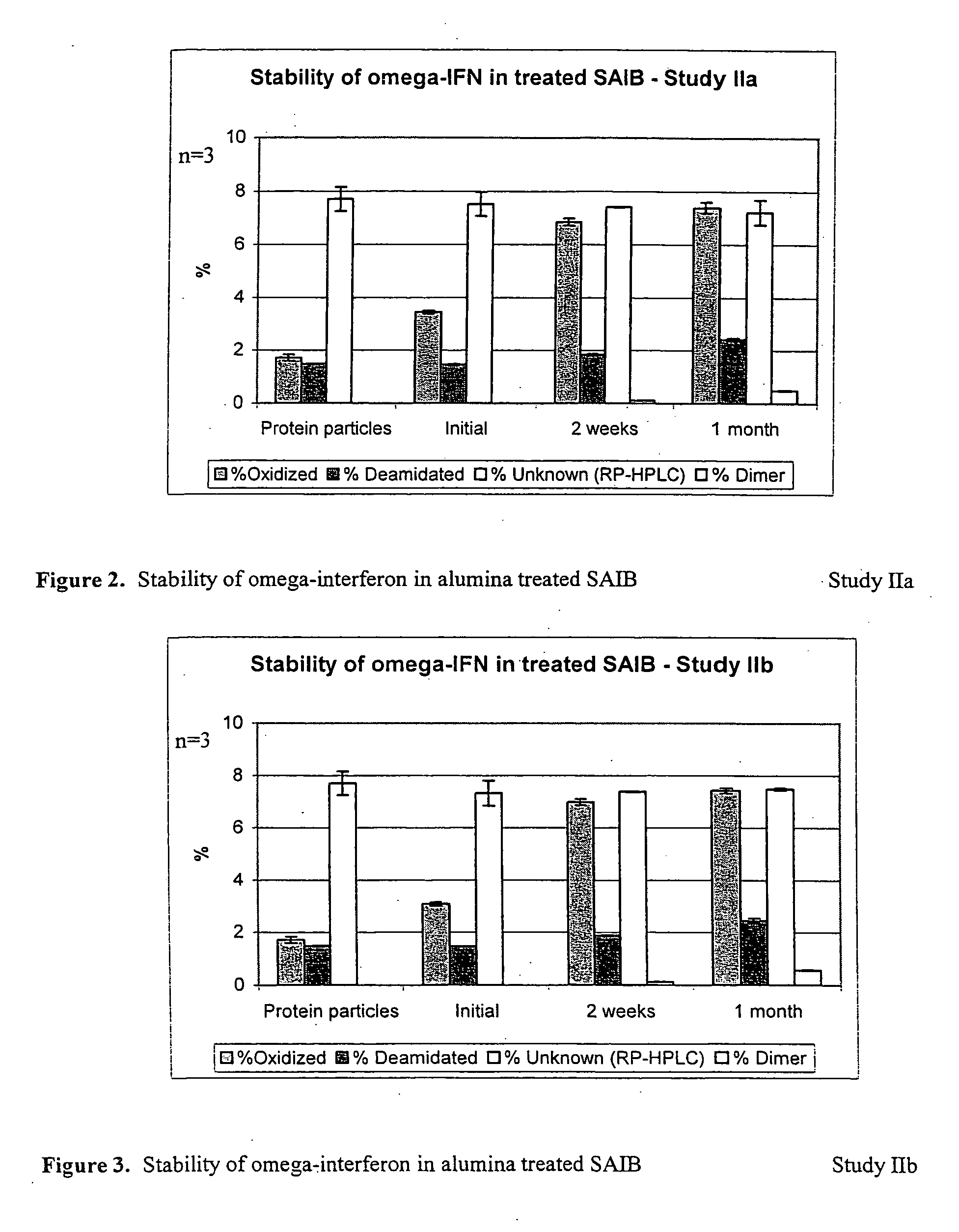
Study IIa and IIb: Stability of SAIB (Lot #TD1030507) Treated with Neutral Alumina with Heating or Neutral Alumina in Presence of Ethanol for 4 Weeks
Treatment of SAIB with Neutral Alumina with Heating
[0044] SAIB was heated to 75° C. Alumina (15% w / w) was added to the heated SAIB. The mixture was stirred for 40 minutes and filtered though a 5.0 μm filter at 75° C. The treated SAIB was then collected, sampled for peroxide testing, and used for preparation of suspension for stability testing.
Treatment of SAIB with Neutral Alumina in Presence of Ethanol
[0045] SAIB was mixed with 15% absolute ethanol to reduce the viscosity. Basic alumina (15% w / w) was added to the SAIB containing ethanol. The resulting mixture was stirred for 1 hour and filtered though a 0.2 μm filter. The filtered SAIB was placed overnight under vacuum at 60° C. to remove the ethanol. This treated SAIB was then collected, sampled for peroxide testing, and used for preparation of suspension for stability testing. ...
example 3
[0047] Study III: Stability in Untreated SAIB (Lot #TD2032663) for 2 Weeks
TABLE 6Stability of omega-interferon in untreated SAIB ((lot #: 2032663) - Study IIIAnalysis by RP-HPLC (n = 3)**Initial (t = 0)(AR 48217Initial (t = 0)1 week2 weeks(protein particles)AR 49640AR 49644AR 49647Assay (%)NA1.69 (0.01)1.70 (0.00)1.68 (0.01)% omega-IFN Purity88.98 (0.09) 88.21 (0.03) 84.95 (0.58) 83.71 (0.48) % Oxidized1.63 (0.04)3.20 (0.03)6.39 (0.05)7.21 (0.10)% Deamidated1.45 (0.01)1.66 (0.01)1.45 (0.40)1.84 (0.03)% Unknown7.94 (0.12)6.93 (0.04)7.22 (0.45)7.24 (0.45)Analysis by SEC (n = 3)**Initial (t = 0)(AR 48217)Initial (t = 0)*1 week2 weeks(protein particles)AR 49640AR 49644AR 49647% Monomer99.93 (0.01) 99.83 (0.02) 99.75 (0.01) 99.51 (0.01) % Dimer0.07 (0.01)0.17 (0.02)0.25 (0.01)0.49 (0.01)UnknownNDNDNDND
ND = Not detected
*n = 6
**standard deviation in parenthesis
[0048] Stability of omega-interferon in untreated SAIB was again tested. The results of a two week stability study (Study III)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com