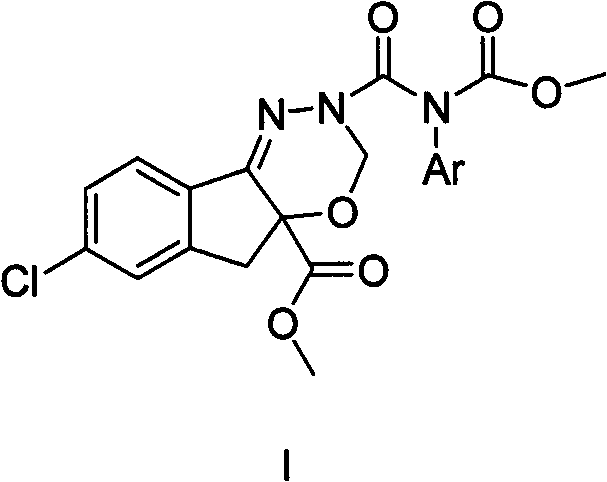
N-substituted dioxazine compound as well as preparation method and application thereof
A technology for oxadiazines and compounds, which is applied in the field of N-substituted oxadiazines and can solve the problems of long residual time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] The preparation method of the compound of general structural formula (I) of the present invention comprises following two reactions and carries out, and reaction formula is as follows:
[0101]
Embodiment 1
[0103] (S)-7-Chloro-3,5-dihydro-2-[[methoxycarbonyl(pyridine)amino]formyl]indeno[1,2-e][1,3,4-]oxadi Preparation of Ozine-4a(3H)-Carboxylic Acid Methyl Ester (SIOC-Y-001)
[0104] Add methyl acetate (20ml), 10% Pd / C (50mg) and 2-(benzyl)-7-chloroindeno[1,2-e][1,3,4]oxadioxane in a 50ml reaction flask Oxyzine-2,4a(3H,5H)-dicarboxylic acid 4a-methyl ester (A) (200mg, 0.5mmol), react under hydrogen protection for 30 minutes; add (chlorocarbonyl)(2-pyridine)carbamate methyl ester (130mg, 0.6mmol) in methyl acetate solution (10ml), add N,N-diisopropylethylamine (200mg, 1.5mmol) and remove the hydrogen protection at the same time, replace the nitrogen protection, react for 12-18 hours; remove Protected under nitrogen, washed with water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, concentrated, and separated by silica gel column chromatography to obtain 161.1 mg of a light yellow solid with a yield of 69.5% and a melting point of 150-152°C.
[0105] Simi...
Embodiment 2
[0106] Example 2. (S)-7-chloro-3,5-dihydro-2-[[methoxycarbonyl (naphthalene)amino]formyl]indeno[1,2-e][1,3,4 -] Preparation of oxadiazine-4a(3H)-methyl carboxylate (SIOC-Y-002).
[0107]
[0108] Melting point: 81-83°C.
[0109] 1 H NMR (300MHz, CDCl 3 )δ: 8.14(d, J=7.5Hz, 1H), 7.96-7.83(m, 2H), 7.66-7.44(m, 4H), 7.36-7.25(m, 3H), 5.70(d, J=9.0Hz , 1H), 5.26(d, J=9.6Hz, 1H), 3.76-3.64(m, 6H), 3.48(d, J=16.5Hz, 1H), 3.27(d, J=16.8Hz, 1H).
[0110] 3. (S)-7-chloro-3,5-dihydro-2-[[methoxycarbonyl[2-(4-methylpyridine)]amino]formyl]indeno[1,2-e] [1,3,4-]oxadiazine-4a(3H)-methyl carboxylate (SIOC-Y-003)
[0111]
[0112] Melting point: 169-171°C.
[0113] 1 H NMR (300MHz, CDCl 3 )δ: 8.17(d, J=4.8Hz, 1H), 7.43(s, 1H), 7.38(d, J=8.7Hz, 1H), 7.30-7.22(m, 2H), 6.89(d, J=5.1 Hz, 1H), 5.82(d, J=9.9Hz, 1H), 5.24(d, J=9.6Hz, 1H), 3.74(s, 3H), 3.70(s, 3H), 3.45(d, J=16.5 Hz, 1H), 3.22 (d, J = 16.2 Hz, 1H), 2.36 (s, 3H).
[0114] 4. (S)-7-chloro-3,5-dihydro-2-[[methoxycarbony...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com