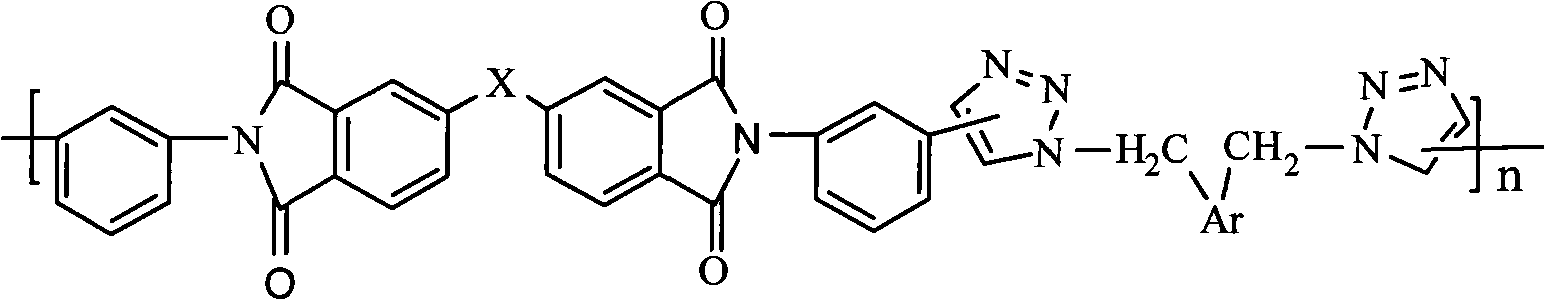
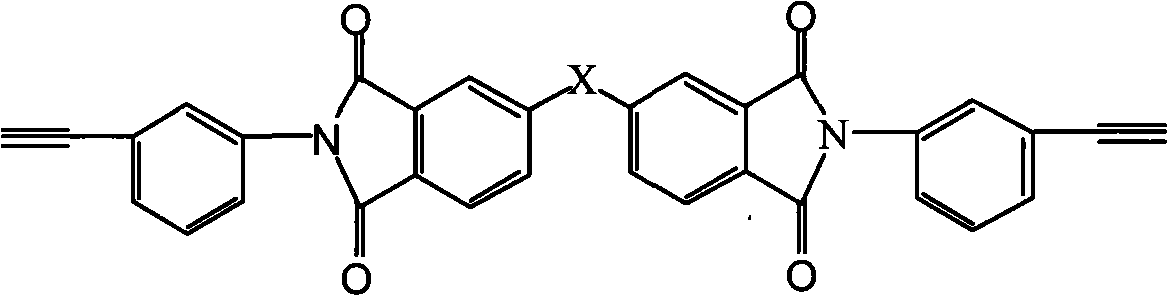
Novel polytriazoles imide resin and preparation method thereof
A technology of polytriazole imide resin and polytriazole imide is applied in the fields of chemistry, chemical industry and materials, and can solve the problems of method limitation of polycondensation reaction, hindering the development of polyimide, and difficulty in synthesis, etc. Mild and controllable, avoiding high-temperature imidization and its side reactions, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) Synthesis of 1,4-diazidemethylbenzene (A1)
[0070] Add 1,4-dichloromethylbenzene 50mmol, NaN 3 150mmol, 20ml of toluene and 20ml of DMF, heated to 70-75°C under stirring, and reacted at constant temperature for 3h. After the reaction, cooled the reaction product to room temperature, poured it into 200ml of deionized water, and left it overnight under freezing conditions to precipitate white flakes The crystal was filtered, the filter cake was washed with deionized water, and dried to obtain a white powdery solid with a yield of 90.0%, mp.26.0-27.5°C.
[0071] (2) Synthesis of bis(N-ethynylphenyl)-bisphenol A ether phthalimide (B1)
[0072] Add 20mmol of bisphenol A dianhydride (BPADA) and 40mmol of m-aminophenylacetylene into a 250ml four-neck flask equipped with stirring, constant pressure funnel and spherical condenser, add 50ml of acetone as a solvent, heat and reflux for 6 hours and then add 50ml acetic anhydride, and added 75ml of triethylamine as a catalyst...
Embodiment 2
[0079] (1) Synthesis of 4,4'-diazidomethylbiphenyl (A2)
[0080] In the three-necked flask, add 4,4'-dichloromethylbiphenyl 50mmol, NaN 3 150mmol, 20ml of benzene and 20ml of DMF, heated to 75°C under stirring, and reacted at constant temperature for 3 hours. After the reaction, the reaction product was cooled to room temperature, poured into 200ml of deionized water, and stood overnight. White solid precipitated, filtered, and used for filter cake After washing with deionized water and drying, a white powdery solid was obtained with a yield of 89.0% and a melting point of 67-71°C.
[0081] (2) Preparation of polytriazole imide PTAI-A2-B1
[0082] Add 10mmol of 4,4'-diazidomethylbiphenyl, 10mmol of bis(N-ethynylphenyl)-bisphenol A ether phthalimide and 40ml of DMAc into the reactor to form a transparent solution. After fully stirring and dissolving, raise the temperature to 60°C, then add the catalyst CuSO 4 ·5H 2 O 0.5mmol, sodium ascorbate 1.0mmol and complexing agent tr...
Embodiment 3
[0086] (1) Synthesis of 4,4'-diazidomethyl diphenyl ether (A3)
[0087] In the three-necked flask, add 4,4'-dichloromethyl diphenyl ether 50mmol, NaN 3 150mmol, 20ml of toluene and 20ml of DMF, heated to 75°C under stirring, and reacted at constant temperature for 4h. After the reaction, the reaction product was cooled to room temperature, poured into 200ml of deionized water, and stood overnight. White solid precipitated, filtered, and used for filter cake After washing with deionized water and drying, a white powdery solid was obtained with a yield of 85.0%.
[0088] (2) Preparation of polytriazole imide PTAI-A3-B1
[0089] Add 10mmol of 4,4'-diazidomethyl diphenyl ether, 10mmol of bis(N-ethynylphenyl)-bisphenol A ether phthalimide and 40ml of DMAc into the reactor to form a transparent solution , fully stirred and dissolved, then heated to 60°C, then added the catalyst CuSO 4 ·5H 2 O 0.5mmol, sodium ascorbate 1.0mmol and complexing agent triethylamine 10.0mmol, and stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Water absorption | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com