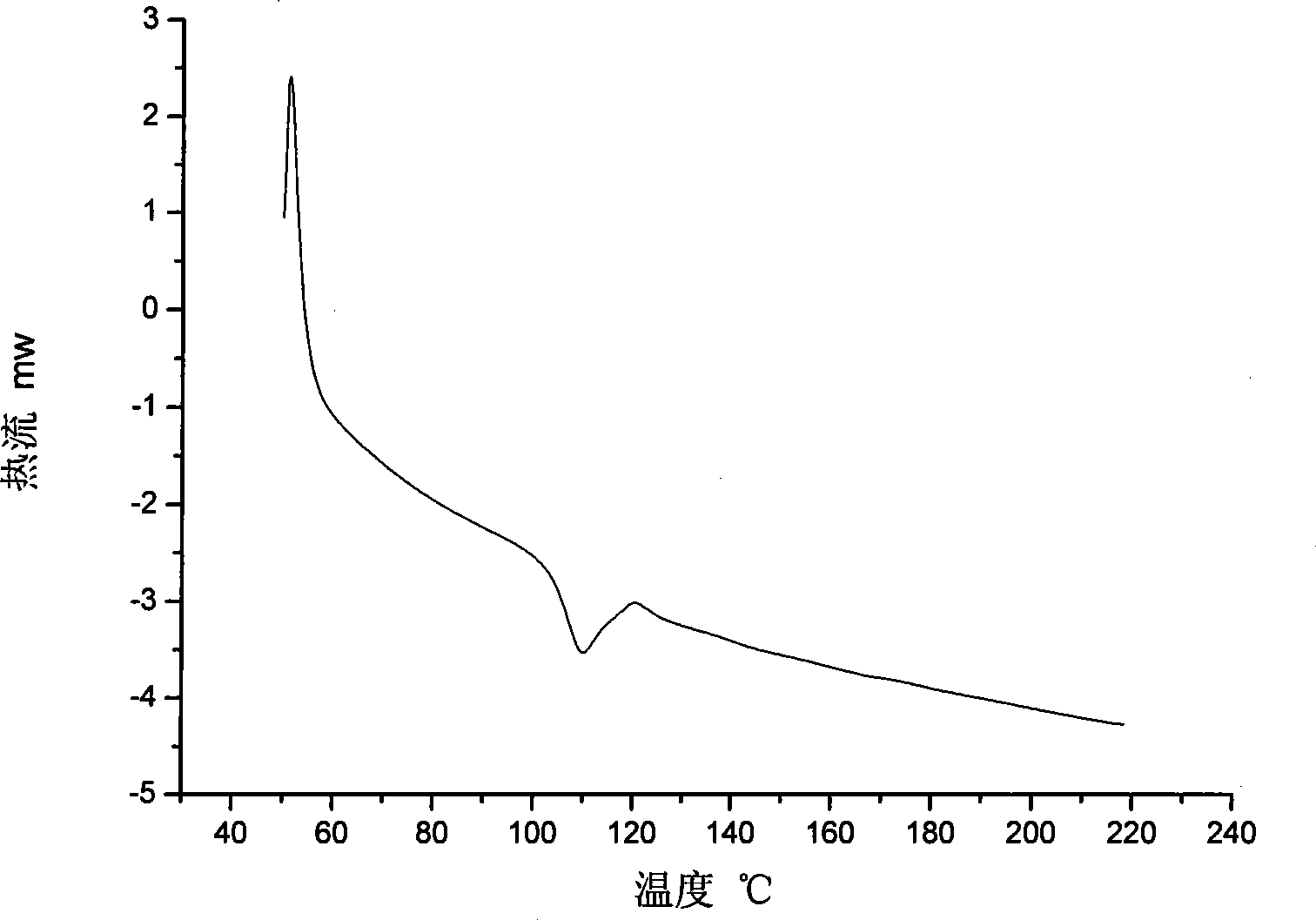
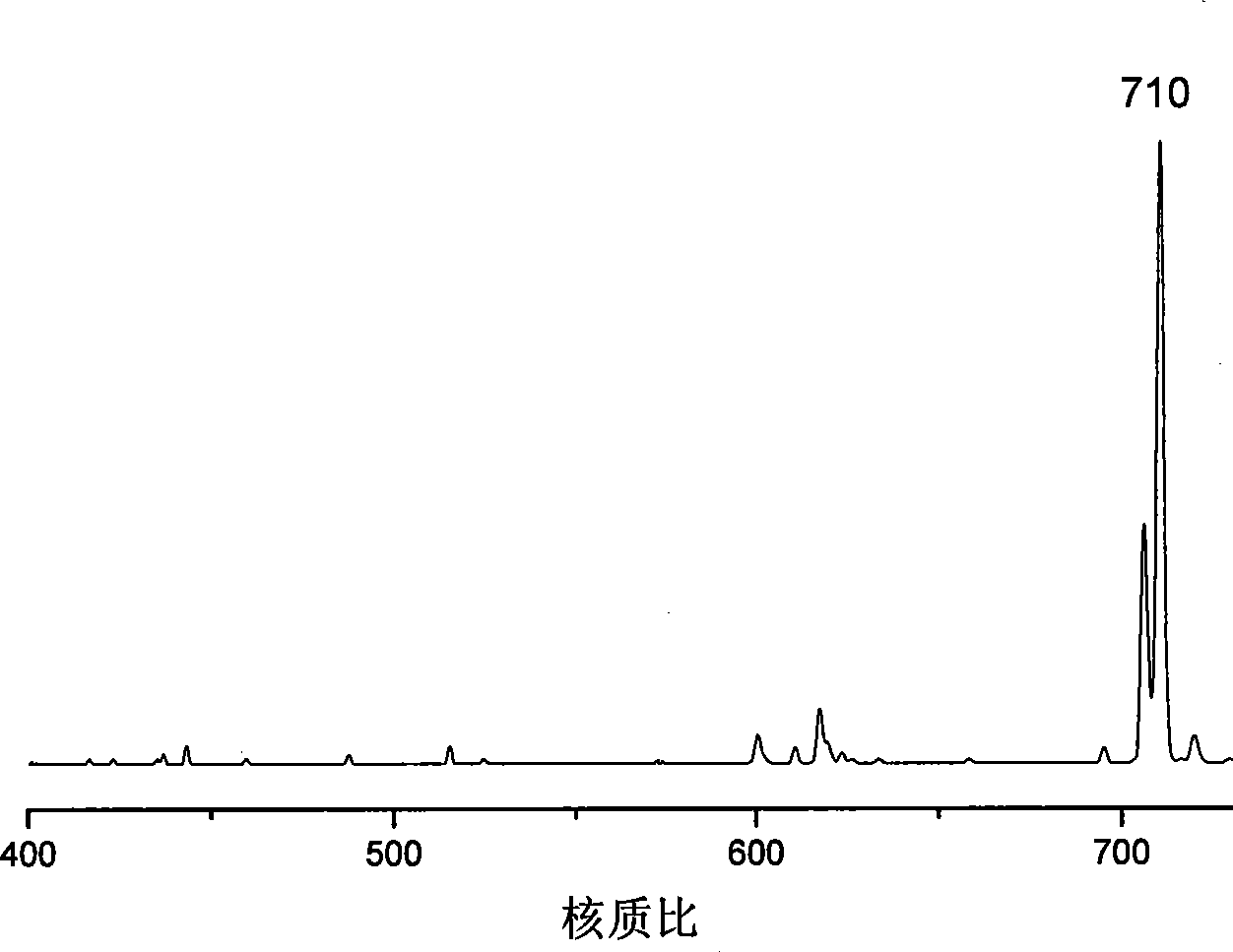
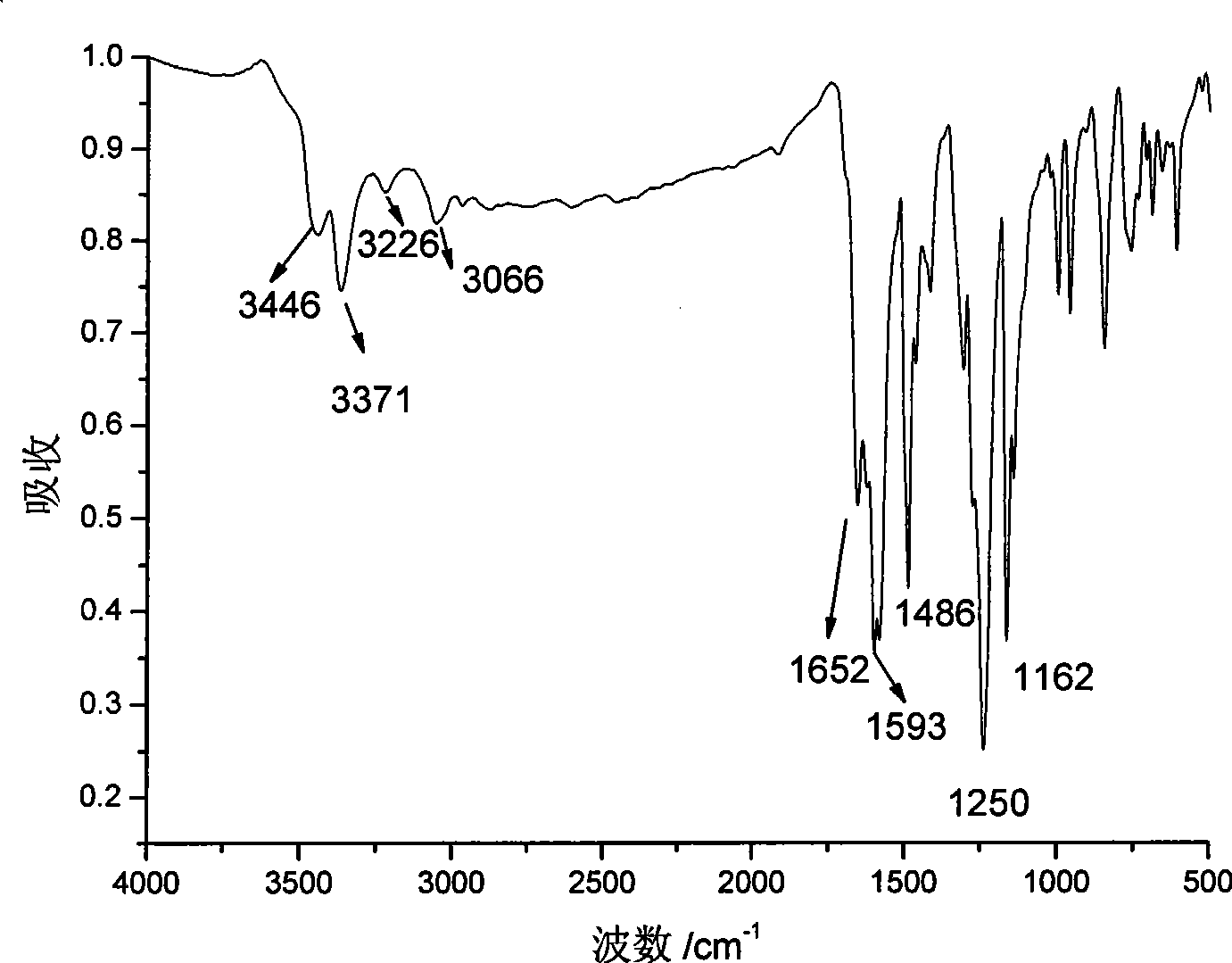
Novel phenylacetylene end capacity capped polyimide prepolymer and preparation method thereof
A technology of polyimide and phenylacetylene, applied in the direction of organic chemistry, can solve the problems of molecular rigidity and high polarity, high molecular binding force, difficult composite materials, high melt viscosity, etc., and achieve wide processing window and high production efficiency. High efficiency and good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: Preparation trimesoyl chloride
[0069] Add 126.0g (0.6mol) trimellitic acid, 256ml (3.6mol) thionyl chloride in the three-necked flask equipped with stirrer, thermometer, dropping funnel, condenser tube, drying tube, tail gas absorption device as solvent, 2ml
[0070] N, N-dimethylformamide DMF is used as a catalyst, and then 511ml (7.2mol) of thionyl chloride is added dropwise to the reaction bottle. After the dropwise addition is completed and stirred for 1.5h, the temperature is raised to 75°C and slightly refluxed for 10h. Acid gas escapes, ending the reaction. Then the water pump decompressed distillation, and thionyl chloride was removed at 59° C. (nitrogen protection during the vacuum distillation process), and 121.6 g of white crystal trimesoyl chloride was obtained, with a yield of 76.3%.
Embodiment 2
[0071] Example 2: Preparation of Trifluoroketone by Friedel's Acylation
[0072] Add 227ml (2.4mol) of fluorobenzene and 80g (0.6mol) of anhydrous aluminum trichloride in a three-necked flask equipped with a stirrer, a thermometer, a dropping funnel, a condenser tube, a drying tube, and an exhaust gas absorption device, and then slowly Add 26.55 g (0.1 mol) of trimesoyl chloride to prevent the reaction from splashing violently. After the addition, continue to stir for 1 h, then raise the temperature to 83° C. and reflux for 6.5 h, then cool down to room temperature and continue the reaction for 9 h. Slowly add the reaction solution to a mixture of 100ml of concentrated hydrochloric acid and 600ml of ice water to remove excess aluminum trichloride, stir while adding, wash with water to neutrality, and use the azeotropic system of fluorobenzene / water to remove fluorobenzene at 80°C Afterwards, the product was dried to obtain 37 g of a crude product with a yield of 83.3%. Take 3...
Embodiment 3
[0073] Embodiment 3: the preparation of triamine monomer
[0074] Add 5.46g (0.05mol) of m-aminophenol, anhydrous potassium carbonate K 2 CO 3 7.60g (0.055mol), 15ml of toluene, 60ml of N,N-dimethylformamide DMF, stirring at room temperature with nitrogen for 10min, then heating, reflux at 134.5°C until no water drops are brought out, then cool the system to 40°C and add trifluoroketone 4.44g (0.01mol), start heating after passing nitrogen gas for 5 minutes, reflux at 134°C until anhydrous, and then release 15ml of toluene, and remove the water-carrying device. The temperature of the system was raised to 147°C, and the reaction was refluxed for 12 hours. Turn off the heating device and lower the temperature to 40°C. After filtering while it is hot, pour the filtrate into a large amount of water and stir to precipitate a red precipitate. Wash the red precipitate repeatedly with water to obtain the crude product, dry it in vacuum at 40°C, and then recrystallize it with ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com