Nickel-manganese composite hydroxide particles, method for producing same, positive electrode active material for nonaqueous electrolyte secondary batteries, method for producing said positive electrode active material, and nonaqueous electrolyte secondary battery
A composite hydroxide and positive electrode active material technology, which is applied in the direction of non-aqueous electrolyte storage battery, secondary battery manufacturing, active material electrode, etc., can solve the problems of selective deterioration of fine particles, high particle size uniformity, and reduced battery capacity. Achieve the effect of being suitable for large-scale production, high particle size uniformity, and high output power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
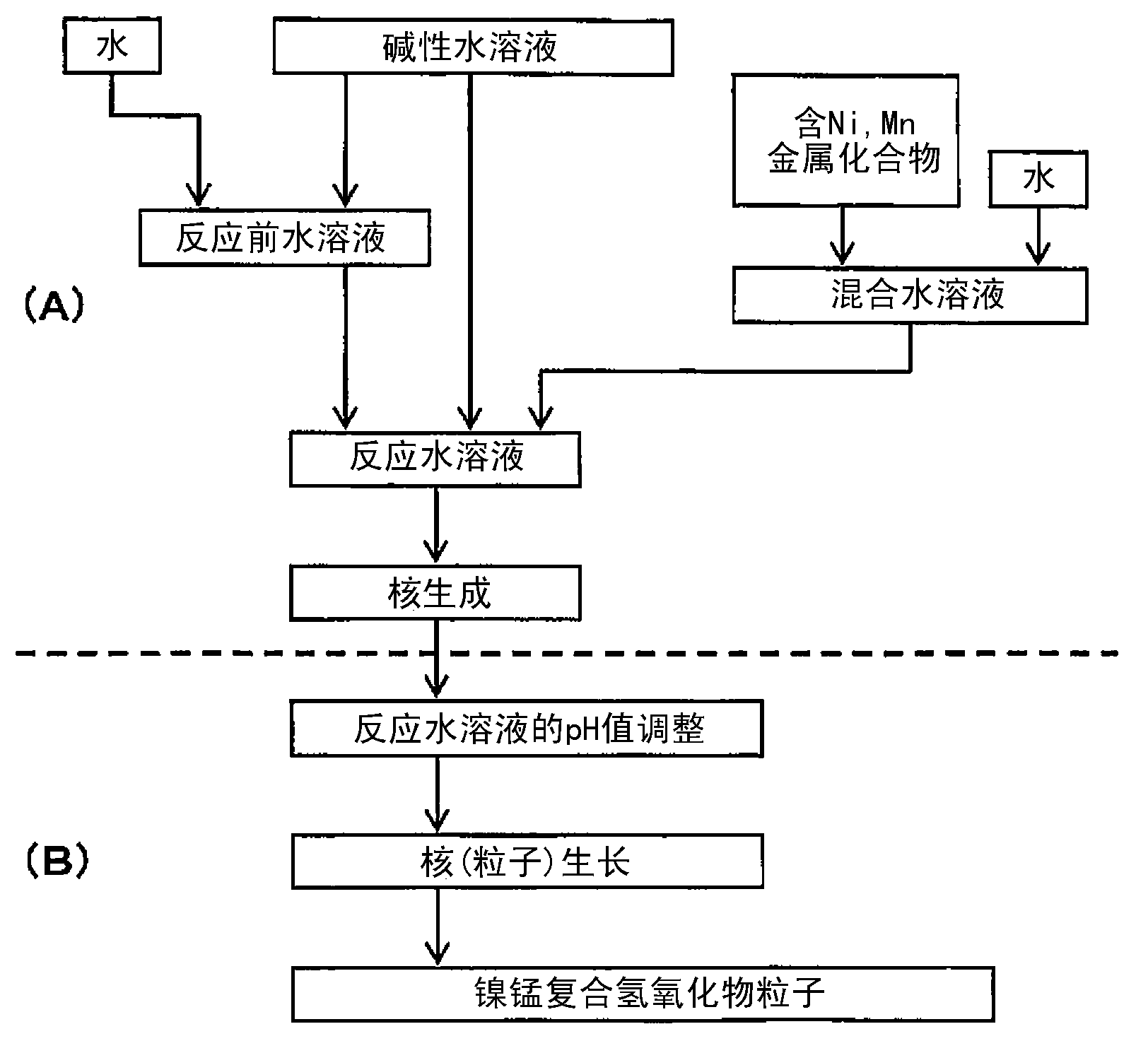
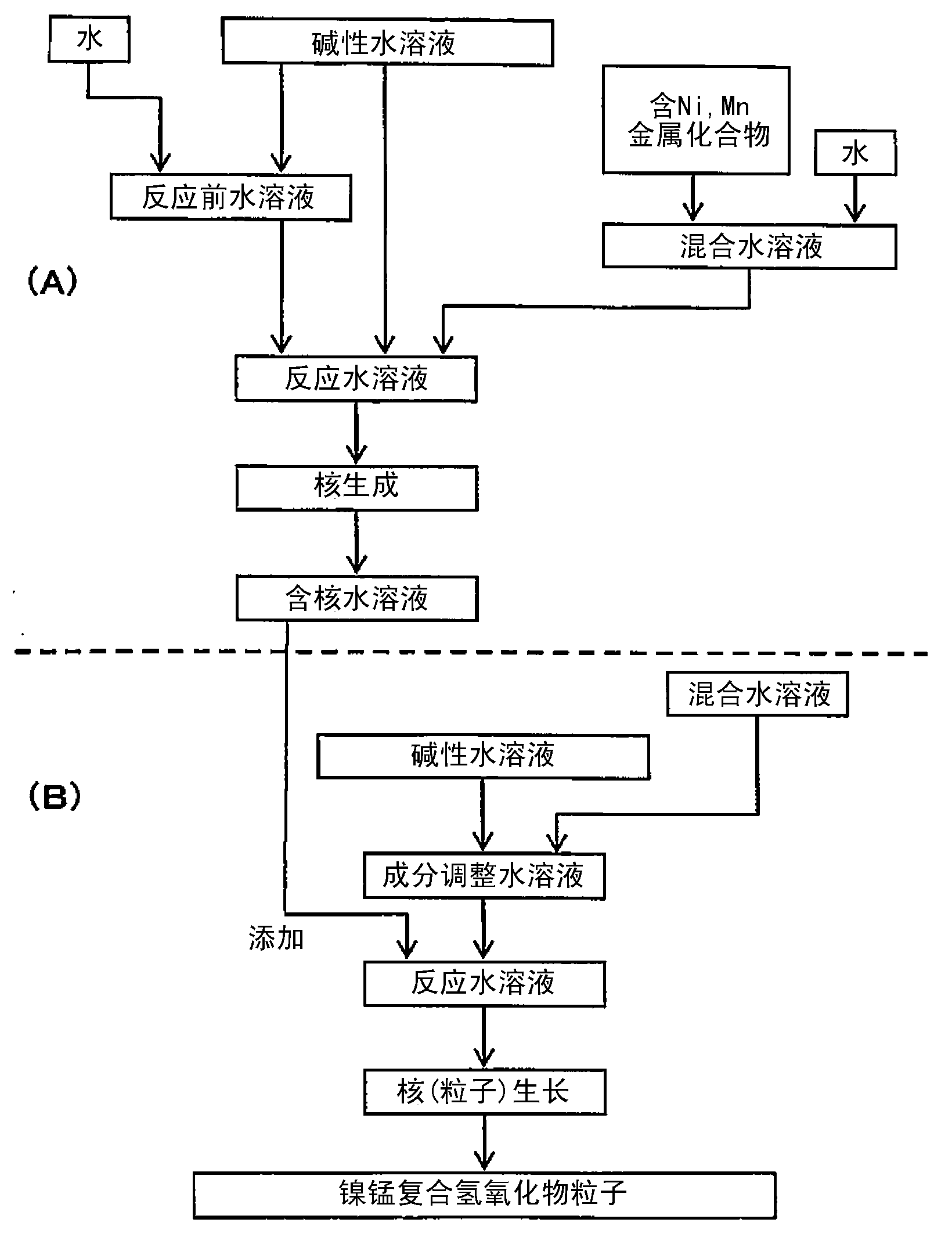
[0263] [Manufacture of composite hydroxide particles]
[0264] Composite hydroxide particles were produced as follows. In addition, in all the examples, reagent special grade samples manufactured by Wako Pure Chemical Industries, Ltd. were used in the production of the composite hydroxide particles, the positive electrode active material, and the secondary battery.
[0265] (nucleation process)
[0266] First, add water to 7L in the reaction tank (34L) and then stir, and set the temperature in the tank to 70°C, let nitrogen flow for 30 minutes, and keep the oxygen concentration in the space in the reaction tank below 1%. The pH of the pre-reaction aqueous solution in the tank was adjusted to 13.1 based on a liquid temperature of 25° C. by adding a 25% by mass sodium hydroxide aqueous solution to water in the reaction tank.
[0267] Next, nickel sulfate and manganese sulfate were dissolved in water to prepare a 1.8 mol / L mixed aqueous solution. In the mixed aqueous solution,...
Embodiment 2)
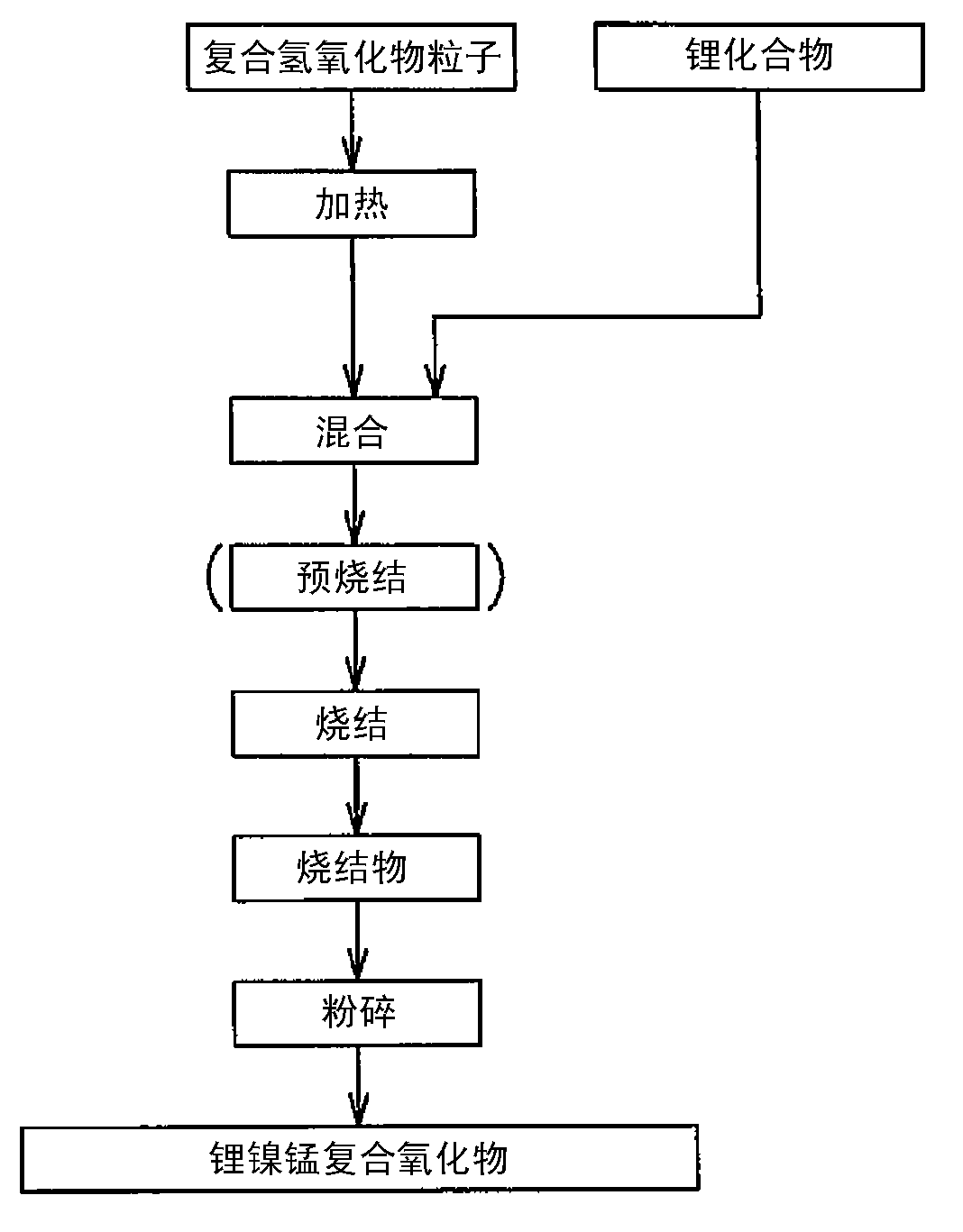
[0302] Except that lithium hydroxide and composite oxide particles were mixed so that Li / Me=1.25, and the sintering temperature was set to 850° C., the same operation was carried out as in Example 1 to obtain a positive electrode active material for a non-aqueous electrolyte secondary battery. .
[0303] The average particle size of the positive active material is 4.8 μm, the [(d90-d10) / average particle size] value is 0.52, and the specific surface area is 1.6 m 2 / g. In addition, by SEM observation, it was confirmed that the positive electrode active material has a substantially spherical shape and a substantially uniform particle size, and has a hollow structure including an outer shell portion formed by sintering primary particles and a hollow portion inside. The thickness of the shell portion of the positive electrode active material determined from the observation results was 0.58 μm, and the ratio of the thickness of the shell portion to the particle diameter was 14.1%....
Embodiment 3)
[0306] In addition to setting the temperature in the tank to 65°C, the pH value of the aqueous solution before the reaction was adjusted to 12.8 based on the liquid temperature of 25°C, and nickel sulfate, cobalt sulfate, manganese sulfate, and zirconium sulfate were dissolved in water to obtain each metal The molar ratio of elements is a mixed aqueous solution of Ni: Co: Mn: Zr = 33.2: 33.1: 33.3: 0.5, and using the mixed aqueous solution of 1.8mol / L, the pH value of the reaction aqueous solution during nucleation is controlled to be other than 12.8. In the same manner as in Example 1, composite hydroxide particles were obtained.
[0307] The composite hydroxide particles are composed of Ni 0.332 co 0.331 mn 0.332 Zr 0.005 (OH) 2+a (0≤a≤0.5), and the average particle size is 3.8 μm, and the [(d90-d10) / average particle size] value is 0.41. In addition, it was confirmed by SEM observation that the composite hydroxide particles were approximately spherical and had a substan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com